Breathing New Life into Existing Drugs
Inhaled administration has the potential to bring benefits to patients – and commercial success to businesses – across a number of therapeutic spaces. But how do you identify the best opportunities for drug repurposing or repositioning?
Finding new uses for old drugs is now a significant trend in the pharma industry. There can be some inconsistency about terminology, but generally speaking, a repurposing project will take a drug already approved for one indication and seek approval for another or, alternatively, a new formulation may be created to allow administration via a different route for the same indication. A repositioning project, meanwhile, tends to take an existing drug and give it an improved or altered product profile, while keeping the same administration route. Either way, as well as providing advantages for patients, repurposing or repositioning can also be used to extend intellectual property (IP) coverage and drive additional revenue for innovators. Drug repositioning now accounts for almost a third of all new drug products, generating half a trillion dollars in annual sales across all dosage forms (1).
Inhaled administration offers significant repurposing opportunities; only a relatively small number of medicines made their first appearance on the market in inhaled form. A number of drugs that are today very familiar in inhalers started life in another delivery format, including beta-2 agonists, anticholinergics, and corticosteroids, as well as antibiotics, such as tobramycin and aztreonam. Though most are locally effective products – notably those designed to treat respiratory disease – some are systemically targeted, such as drugs to treat diabetes, migraine, or Parkinson’s disease.
Repositioning is more likely to reflect lifecycle management strategies, whether by providing a new improved inhaled delivery mechanism, or offering patients improved dosing regimens. It is also possible to use the inhaled route to dose more than one drug at the same time, opening up opportunities for new combination products. Alongside the actual drug and its formulation, an improved delivery device can also encourage enhanced patient adherence.
Faster and cheaper
Repurposing or repositioning a drug is often a faster and more affordable way to develop new products than de novo drug discovery and development. Attrition rates are lower, as the active will already have passed safety trials, and less preclinical testing is required, with inhaled toxicology the main tests potentially needed. The reduced timescales needed for the project also translate to lower costs.
Inhaled products can offer therapeutic advantages over oral or injected dosage forms, even if they are not used to treat diseases of the lung. One of the most important benefits that the inhaled route offers is fast onset of action. If the molecule is inhaled sufficiently deeply into the lungs, the speed with which it is taken up by the bloodstream is almost as fast as administration by injection. This rapid uptake has been exploited with orally inhaled insulin products, such as Affrezza (MannKind) and Pfizer’s now-withdrawn Exubera, as well as with products aimed at treating pulmonary arterial hypertension, such as Bayer’s Breelib (iloprost). Other conditions where speed could be a distinct advantage include pain relief, particularly migraine, and in Schizophrenia, bi-polar disorders, and Parkinson’s disease, where rapid onset would be desirable to treat acute symptoms. Inhaling a drug also avoids first-pass metabolism of the liver that occurs with oral dosing, which can enhance the activity of a drug that may be broken down quickly through hepatic metabolism or by the digestive system. Avoiding this metabolism may also make beneficial changes to the metabolite profile, which is particularly important if a drug has known metabolite-related adverse events.
On the life-cycle management front, a drug approved for an adult might undergo repositioning for the pediatric market, and this may or may not include changing the delivery mechanism; for example, from a pressurized metered dose inhaler (pMDI) to a smart nebulizer or a dry powder inhaler (DPI) to suit the expanded patient population. Children may find it particularly difficult to synchronize inhalation with a pMDI and may have less user errors with a DPI or nebulizer presentation.
Of course, there also has to be a therapeutic rationale for the change. The starting point should, therefore, be to determine an unmet therapeutic need, and how market share and commercial value might be derived from meeting it. For innovators, improving patient compliance is important because if patients take a drug correctly and therapeutic benefits are established, it will likely result in more prescriptions being filled. In inhalation, there is ample opportunity to improve upon the delivery method. For example, metered dose inhalers can be difficult for patients to use correctly because of the requirement to time the dose with breathing in, so advanced devices that improve patient use and compliance are important. An innovative delivery device can also provide an additional layer of market protection from competition.

Figure 1. Patient prevalence in the respiratory market. Sources: WHO, British Lung Foundation, GSK, BI.
Identifying the right niche
Looking more closely at clinical need, the global respiratory market obviously continues to be the most attractive. Significant unmet patient needs remain in asthma and chronic obstructive pulmonary disease (COPD), as well as more niche diseases, such as cystic fibrosis, pulmonary hypertension, and idiopathic pulmonary fibrosis, as shown in Figure 1, with some low volume but high value opportunities. Increasingly, the strongest growth potential lies in rarer diseases and systemic indications. The general market is growing at a rate of about 6 percent. But with cystic fibrosis, for example, the market is growing more quickly at >10 percent (see Figure 2). Drug designation for rare diseases can also come with other advantages, such as potential fast track designation, abbreviated approval processes, accelerated review, tax credits, fee waivers, and extended exclusivity – depending on the market region and regulator.
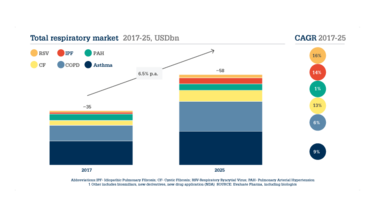
Figure 2. Total respiratory market. Data taken from EvaluatePharma.
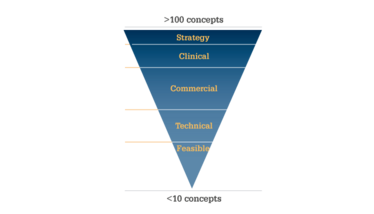
Figure 3. Key considerations in selecting an
asset to develop.
Systemic diseases, where delivery via the lung can benefit or enable targeting, offer substantial opportunities for drug repurposing. Drugs can be given a faster onset of action, while conserving potency, safety and tolerability. It can also be possible to dose within tight therapeutic windows, or to mimic the pharmacokinetic profiles that are achieved with the injectable drug delivery route. Inhaled administration can also be potentially used to effect mucosal vaccination.
A formalized and rational process should be used to identify the repurposing or repositioning projects most likely to succeed. The process should evaluate a product’s potential against key criteria, to filter opportunities and short-list product concepts. The evaluation should consider business strategy, clinical needs, commercial opportunity, technical aspects, and overall feasibility (see Figure 3). As an example, there are an estimated 300 projects within development databases that could benefit from an inhaled dosing strategy. The first step should be a quick triage of potential projects’ alignment to corporate vision and R&D investment strategy. This should still leave a considerable number of projects – perhaps around 100. The next stage of the filter is to consider clinical needs and market potential. An unmet medical need provides a compelling value proposition. Even if the condition is already covered by other products, the size of the patient population and the prevalence of the disease may still make the project viable. However, there should be a sound scientific rationale alongside the efficacy, safety and mechanism precedent.
The next consideration is commercial potential. It is important to judge how crowded the existing marketplace is, and whether a new product is sufficiently differentiated against the competition to offer both clinical and commercial value. Without these, it is unlikely that the drug will gain sufficient market share and meet reimbursement targets. Assessments also need to be made as to whether the desired value inflexion could be achieved within a reasonable timeframe, and whether the predicted return on investment is sufficiently attractive.
A commercially viable project will also need technical advantages. In-house proprietary technology, intellectual property, and improved functionality can all drive meaningful market differentiation. These aspects are also likely to provide product protection to deter direct competition.
The final filter is a project’s feasibility. Successful inhaled delivery is notoriously complex to achieve, and there are barriers to entry from a practical perspective; can the drug be formulated and delivered as an inhalable aerosol, and can it be dosed consistently in sufficient amounts at the target site of deposition within the lung to be efficacious and safe? Research and detailed diligence should be undertaken to assess whether a project leverages a company’s specific technical know-how, as well as the disease area and geographic focus. This final filter should leave just a handful of potential projects that meet all criteria.
De novo drug development is expensive and time consuming, so repurposing and repositioning existing drugs for new applications is extremely appealing from a commercial perspective. However, it’s crucial that the evaluation process also considers the scientific rationale and how patients will benefit. There is particularly unmet medical need in lung diseases, both common and rare, and the opportunity to improve on drug delivery methods through more advanced devices for both asthma and COPD, but the opportunities don’t stop with respiratory diseases – there is plenty of potential in the systemic delivery space too.
Examples of Differentiation and Improved Adherence
New technology – that benefits patients – is one way to differentiate a product from the competition. As an example, nebulizer devices have been designed to improve drug delivery using flow and volume control to better target the nebulized aerosol to the correct part of the lung, or within a specific type of inhalation profile. Modern devices are also breath actuated, which means they only nebulize while the patient is actively inhaling, and can also be Bluetooth-enabled so that treatment can be monitored via an app or a web portal to check adherence.
As a case in point, Vectura developed Breelib with Bayer, which was launched in 2017. This is a repositioned version of iloprost (Ventavis), which is used to treat pulmonary arterial hypertension. Without treatment, life expectancy is about three years, but with iloprost, this can often be extended to 10–12 years. However, the drug needed to be dosed nine times a day via a nebulizer, and each dose took 10 minutes to administer. Clearly, there was an opportunity for improvement.
Repositioning the drug into a Fox nebulizer allowed the dosing time to be reduced to three minutes through more efficient mesh nebulization with inhalation flow rate and volume control. Although this must still be done up to nine times a day, the total time spent dosing each day is reduced to under half an hour. The result is a substantial improvement in quality of life for the patient (2). The device also gives the patient feedback, leaving them feeling more in control of their treatment and their disease.
Another example is Mylan’s TOBI (inhaled tobramycin) – a narrow therapeutic index aminoglycoside antibiotic used in the treatment of Pseudomonas aeruginosa lung infection in cystic fibrosis (CF) patients. Tobramycin was originally administered by intravenous or intramuscular injection before being repurposed as an inhaled nebulized therapy. Local lung delivery by nebulization in CF is clinically proven to improve respiratory function, decrease hospitalizations and reduce the need for systemic antibiotic use. The latter is prone to induce adverse side effects. Some of these, such as nephrotoxicity and ototoxicity, are irreversible (3).
The TOBI Podhaler is a lifecycle extension of the marketed nebulizer product. This dry powder inhaler is a repositioned product, which reduces treatment burden whilst improving patient convenience. It achieves this by providing patients with a room temperature stable formulation, using a portable inhaler with no requirement for a power source or compressor. Administration time is also reduced from about 20 minutes to five minutes twice a day, whilst also eliminating the need to clean and disinfect the nebulizer. The beneficial impact on the CF patient’s quality of life is significant in terms of their freedom from lengthy home administration of essential antibiotic therapy. And that may translate into better treatment cycle adherence and clinical outcomes (4).
- S Naylor et al., “Therapeutic drug repurposing, repositioning and rescue,” Drug Discov (World Spring Edition 2015).
- T Gessler et al., “The safety and pharmacokinetics of rapid iloprost aerosol delivery via the BREELIB nebulizer in pulmonary arterial hypertension,” Pulm. Circ., 7, 505-513 (2017).
- BW Ramsey et al., “Intermittent administration of inhaled tobramycin in patients with cystic fibrosis,” N Engl J Med, 340, 23-30 (1999).
- DE Geller et al., “Development of an inhaled dry- powder formulation of tobramycin using PulmoSphere technology,” J Aerosol Pulm Drug Deliv, 4, 175-182 (2011).


















