Why We Like Virus-Like Particles
Could Hepatitis E breathe new life into old drugs in the fight against cancer?
The side-effects of conventional cancer therapy can be devastating. Collateral damage is inevitable with treatments that attack all rapidly dividing cells, which is one reason why targeted biological therapies, such as antibody-based immunotherapies, are gaining traction. But there may be another option... Marie Stark, graduate student at University of California, Davis, USA, and her colleagues in the Department of Molecular and Cell Biology believe that Hepatitis E particles could eliminate the scatter-gun effect of most chemotherapies, and allow older drugs to be used in a less damaging way. By replacing the DNA cargo of the viral particle with a cancer drug and adding a cancer-targeting molecule to the particle surface, they have created the potential for a magic bullet. The group has already shown that their virus-like particles can hone in on breast cancer cells, both in vitro and in a mouse model (1). We spoke with Marie to find out more.
Why Hepatitis E?
Professor Cheng’s group was initially working on Hepatitis E Virus-Like Particles (HEV-VLPs) as a potential vaccine delivery system. It became clear that the Hepatitis E virus capsid had unique attributes – for example, native Hepatitis E infects humans through the fecal/oral route and is therefore resistant to acidic and degradative conditions of the gastrointestinal system. This stability is a function of the capsid – so, our VLPs, which in effect are just hollow capsids, also are stable during gastrointestinal transit. Such stability under environmental extremes suggested the utility of HEV-VLPs beyond the vaccine field. We undertook further structural investigations, including isolating, modifying and expressing the capsid protein, to develop a useful delivery system. I should emphasize that HEV-VLPs are derived from structural protein sequences only – they are devoid of infectious, replicating elements of the virus. Accordingly, HEV-VLPs capsids benefit from the structural stability of the Hepatitis E virus, while avoiding any possibility of virus infection.
What makes the HEV-VLP useful in drug delivery – particularly cancer drug delivery?
Cancer therapies are trending towards improved targeting and stable circulation methods, such that cancer treatments can attack tumors while minimizing harm to healthy tissues. HEV-VLPs not only provide a platform that can be modified to target a variety of cancer cells, but also serve as vehicles for delivering cancer drugs. In this way, a tumor-targeted HEV-VLP can be packaged with drug – or nucleic acid, if a gene therapy approach is used – and deliver it to the tumor site. One could envisage adding magnetic nanoparticles to the HEV-VLP cargo to provide an additional layer of targeting via an externally applied magnetic field. In any case, the consequence of our approach is that cancer therapies would not broadly circulate in healthy tissues; rather, a specific dose would be packaged in the VLPs, delivered systemically, and released locally at the tumor site, reducing the potential for side-effects.
How do you target the VLPs to cancer cells?
The protrusion domain on the HEV-VLP capsid sequence was genetically modified with a cysteine residue that acts as a site for attaching cancer-targeting molecules via a simple covalent chemical reaction. Consequently, the VLP displays targeting molecules that can bind to tumor cell ligands, while being protected from degradation by the capsid structure.
While this study specifically tested LXY30, which targets breast cancer cells, the system is compatible with virtually any functionalized targeting molecule, furthering the trend towards personalized targeted cancer treatment.
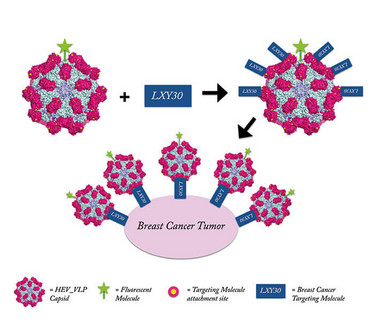
Have you already considered scale-up?
The ability to scale up was a key aim in optimizing the HEV-VLP delivery system. We produce HEV-VLPs from an FDA-approved insect cell amplification system already in use for clinical-grade manufacture, such as vaccine production. We modified the capsid structure so that it is excreted into the supernatant following capsid assembly, resulting in high yields and easy purification. Because of their stability, HEV-VLPs can be stored in high concentrations under simple storage conditions. The modified attachment site on the VLP is particularly useful, because the VLP can be readily activated with any functionalized cancer-targeting molecule. This post-production attachment capacity is very significant – it means targeting molecules can be bound to HEV-VLPs via a simple chemical reaction, post-production. You don’t need to return to the early stage HEV-VLP amplification step, if you want to change the VLP affinity.
What’s the next step?
As noted, the modified HEV-VLP not only has the capacity to target specific cells, but can also encapsulate genes, metallic nanoparticles, and drugs. To that end, we are currently investigating various avenues of encapsulation.
- H.Cheng et al., “Chemically activatable viral capsid functionalized for cancer targeting,” Nanomedicine, 11, 4, 377-390, (2016). PMID: 26786134


















