Let the Molecule Decide
Formulation techniques and technologies can easily become more habit than science, but a single technology does not work for all compounds. Each new drug is unique and deserves a fresh approach to formulation.
Every molecule – large and small – has a structure that drives its physicochemical and biopharmaceutical characteristics – and its potential as an active ingredient. The smoothest path to market lies in understanding your molecule’s characteristics and applying strategies to overcome any deficiencies as early as possible. With sound expertise and judgment, physicochemical properties, such as the molecule’s solubility, permeability, and stability, can be evaluated at the early stages of development – and from there, you can make decisions about the best formulation approach and what the molecule needs to help it become a commercial success.
Of all the decisions made at the early preclinical stages of oral drug development, there are two that are perhaps the most fundamental. The first is to select the nature of the potential API and its form (salt and polymorph), which requires proper physical characterization and optimization of the API manufacturing process. The second is to select an appropriate drug delivery system, with improved solubility and bioavailability usually being key criteria.
Decisions, decisions
Given that most molecules in development are poorly soluble, companies often choose to use a solubility enhancing technology, but which technology is best? Knowledge in the field is growing constantly and today there are many solutions available to help overcome solubility and bioavailability issues. Since each molecule is unique, the challenge is to collect sufficient information in the very early stages of development to support decision making, without consuming precious time and often limited API.
In an ideal world, data are always conclusive, and – if delays are to be avoided – available when a decision is needed. For example, it is not typical for the final API manufacturing process to be established and scaled up prior to drug delivery system selection. It is therefore necessary to consider the fact that future API changes could impact the drug delivery system. In the real world, data tend only to be collected in response to specific requests and may not be available – or may be inconclusive. Because of the sheer number of decisions required to bring a drug product to market, there are frequent occasions where data are not available or where resource or time consuming errors delay programs. In early development, API is typically scarce so it is important to minimize the number of tests as much as possible (without jeopardizing data integrity). If this is done effectively, the most common reasons for drug development attrition (poor pharmacokinetics, toxicity, and poor efficacy) can usually be addressed at the outset.
There is a tendency for formulators to favor a particular drug delivery technology that they have experience with and to pair it with almost every molecule they work with – but no technology is a panacea. A better way of approaching drug development is to pay close attention to the physicochemical data and what it is telling us about the molecule, and to approach formulation selection with an open mind. What is really best for the molecule? What will help it to reach its full potential? We need to let the unique attributes of the molecule dictate our decision, rather than simply choosing the same path that has worked before. Just because something has worked once does not mean it will work well again.
Knowledge is power
The starting point for an early formulation project is an API-sparing, cost-effective screening program to determine and quantify the most common parameters that affect the drug molecule’s behavior both in-vivo and in the final dosage form, such as purity, solubility, stability, hygroscopicity, melting point, particle size, and excipient compatibility. During these tests, it is important to look for evidence of potential issues and to recommend the collection of more comprehensive data where warranted (for example, from a second lot of the same API). Building a drug molecule’s profile helps to determine the development strategies that are feasible, those that are not feasible, and those that are essential.
Two of the most important pieces of information typically collected for each API are its ability to dissolve when dosed to a patient and its ability to permeate across the gut wall into the blood – these data are used in both the biopharmaceutics classification system (BCS) and the developability classification system (DCS). Only a small proportion – less than 10 percent – of molecules in the development pipeline are thought to have both adequate solubility (dose-solubility ratio> 500 ml) and permeability (> 1 x 10-4 cm/sec) to achieve good (>90 percent) bioavailability (1). Solubility can be addressed during both API form selection and drug delivery technology selection. During API development, solubility and stability can be improved by chemical modification or by optimizing the physical form of a drug molecule’s salt and polymorphic form (including co-crystals). In addition to changing from one polymorphic form to another, solubility can also change for a single form when only purity changes. Early on in development, there may be multiple molecule candidates to choose from. As traditional methods to detect and generate salt forms, co-crystals, and polymorphs demand significant time and API, you have to make a difficult choice: do you proceed without the data or do you complete some of the evaluation with novel methods, such as material-sparing, high-throughput screening (HTS) techniques and in silico screening? Though HTS has greatly increased its ability to cover a larger number of samples in recent years, care must be used in the interpretation of such small-scale experiments. With any in silico or high throughput screening, there is always a concern that the model can only be truly validated once a full data set is compiled.
If the selected API candidate has poor solubility, stability or purity, and is easily ionizable, salt forms may be used to improve one or more of these properties. There are numerous pharmaceutically acceptable salts (hydrochlorides, tartrates, succinates, acetates, and so on) that can be chosen, but a salt form may not be a viable option for drugs that lack an easily ionizable function group. In these cases, co-crystallization could be used to improve chemical stability, hygroscopicity, solubility, dissolution rate, and bioavailability. Co-crystals are non-ionic supramolecular complexes between the drug molecule and a co-former. There are a number of established co-formers that can provide a starting point for a screening campaign.
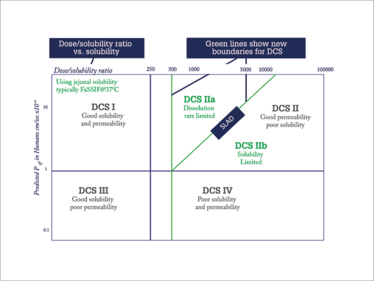
Figure 1. A molecule’s position on the DSC plot can help guide the formulator in terms of which drug delivery technologies are most likely to produce a successful product.
Regardless of whether the molecule is a pure form, salt, or co-crystal, the US FDA and International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) require identification and characterization of all polymorph crystal forms. Polymorphs are crystals with the same chemical composition, but possess different lattice structures and/or different molecular conformations. Polymorphs have different physicochemical properties and will behave differently (think of carbon as both graphite and diamond). Although a polymorph may have higher solubility, it is likely metastable and will typically convert to the more thermodynamically stable (and less soluble) form over a short time. It is crucial to ensure that the best polymorph is selected and formed every time during manufacture – and that it remains stable during storage.
Finding a formulation
Once you have your API information in hand, the next step is to determine the optimal drug delivery technology. DCS is an effective starting point (2) and takes its cue from the molecule itself. The molecule is classified based on its dose-solubility ratio and effective permeability – and its position on the DCS plot (Figure 1) will help guide the formulator in terms of which drug delivery technologies are most likely to produce a successful oral product. Like every other data set, DCS classification needs to be updated as more information is gathered, and as confidence in the data increases.
The position in the DCS grid determines the optimal starting point for formulation options. Those in Class I (good solubility and permeability) have a relatively straightforward development path where a solution, suspension, tablet or capsule will work; the choice will hinge on what is likely to be most acceptable to patients and/or the most cost effective – and whether there are any other factors that would prevent the use of a particular technology. Nearly 70 percent of drug molecules in development fall into Class II (good permeability, but poor solubility) and require a solubility enhancing formulation. Class III molecules (good solubility, but poor permeability) are likely to be amenable to suitably designed tablets or capsules, but may require permeation enhancement. Those in Class IV (poor solubility and permeability) may be acceptable in a formulation that includes solubility and/or permeability enhancing technology, providing the target bioavailability is realistic (not 100 percent). What to do with molecules that are deep in Class IV territory? Perhaps it’s better to go back to the candidate API selection or to employ a prodrug strategy.
One of the benefits of DCS classification over BCS classification is that the former splits Class II drug molecules into two sub categories: in DCS IIa, the solubility limited bioavailability results from a limited dissolution rate rather than the actual solubility itself, while in IIb the full dose is not expected to dissolve before exiting the small intestine – the strategy for creating a suitable dosage form differs between the two. And the boundary between the two subdivisions is based upon the solubility limited absorbable dose (SLAD), below which all the dose could dissolve and above which the fraction dissolved will diminish with increasing dose.
This important subdivision of DCS is useful in guiding the formulator to the right type of drug delivery technology. For Class IIa, simple particle size reduction or solutions are a great fit. For class IIb, a lipid-based solution or amorphous solid dispersion might be more suitable. If the molecule falls in IIb close to the SLAD boundary, then co-micronization (with the addition of a suitable surfactant) may be successful.
With a promising range of formulation options in hand, the next step is to evaluate their behavior in pharmacokinetic tests in animals to confirm which performs best under more stringent, in vivo conditions. The data will support the decision on whether to progress to first-in-human Phase I clinical trials, or whether further refinement of the formulation will be required to produce acceptable bioavailability in the clinic.
The final say
The title of this article is “Let the Molecule Decide”, but of course it is not the molecule itself that actively chooses its formulation route – the studies and decisions are made by expert scientists from a range of disciplines who have the ability to separate opinion from fact, and to determine confidence in and the reliability of those facts. When we use the phrase “let the molecule decide,” what we really mean is that we should eliminate the subjective and increase the reliability of data. Doing so will help us to rapidly and effectively get to a point where both the molecule’s nature and its likely in vivo performance are well understood.
Whether it will ever be possible to make these predictions accurately in every case is an ongoing debate. What is certainly true is that scientists are only human and not every development program presents a single clear obvious path and can be conducted with the ideal amount of time and resources. Scientists come from a broad range of disciplines; may or may not have specific qualifications; may or may not be skilled in the analysis of data; and may develop an unintended bias for a particular approach based on familiarity, which can result in molecules being paired with an unsuitable delivery technology. Scientists need to be creative and they need to be able to understand what the data for a given molecule are really “saying”. Ultimately, scientists will make their choices, but it is the molecule that will have the final say when it comes to whether or not it can improve patients’ lives.
Stephen Tindal is Director of Science & Technology, and Ronak Savla is Scientific Affairs Manager, both at Catalent Pharma Solutions, New Jersey, USA.
From Small Acorns...
It would not be unreasonable to expect that every pharmaceutical company has a process for drug development that includes experienced, educated people who are familiar with a wide range of technologies, but this is not always the case. Each company has subtly different goals, skillsets, capabilities and experiences built across the molecules and technologies they have worked with – and some will be better than others, according to a natural distribution. In particular, small companies, which usually have the advantage of being able to make fast decisions, tend not to have access to a large pool of talent, and often cannot afford all of the instruments and equipment that are found in big pharma formulation laboratories. In addition, big companies usually have several molecules in their pipeline and part of their strategy allows for a certain level of attrition, whereas a small company’s entire future may depend on the success of one asset. It is more important for them to be thorough in early development to reduce the risks of failure in later development stages – a number of common issues, such as poor pharmacokinetics or toxicity, can all be predicted in early development with the right tests.
The pharma industry is fortunate to have a f lourishing and wellestablished contract manufacturing field. If a company doesn’t have enough formulation experience or knowledge in house, then there are several contract manufacturers to choose from – some of whom have deep experience gained from formulating hundreds of molecules using different techniques and technologies. With limited amounts of APIs, it is possible to screen API forms and drug delivery technologies together using a combination of in silico screening, high-throughput screening, and parallel technology evaluation. Traditional drug development has relied on selecting a technology early on, at a time when paucity of data may preclude the best selection.
A growing number of drug molecules rest in the pipelines of small companies and they are important players in the pharma field. The industry needs to support these smaller companies by developing accessible outsourcing platforms designed to collect the most relevant data and to gain insight into each molecule’s challenges. Overall, this will result in more molecules reaching the market and a wider selection of options for patients.
- Gl Amidon et al., “A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability”, Pharm Res., 12, 413-20 (1995).
- JM Butler, JB Dressman, “The developability classification system: application of biopharmaceutics concepts to formulation development”, J Pharm Sci., 99, 4940-54 (2010).
Stephen Tindal is Director of Science & Technology, and Ronak Savla is Scientific Affairs Manager, both at Catalent Pharma Solutions, New Jersey, USA.