Follicular Drug Delivery: a Root to Success
Delivering drugs through the skin without the use of needles has proven a significant challenge for the scientific community. But what about exploiting hair follicles?
Hanzey Yasar, Sarah Gordon, Brigitta Loretz, Kai Schulze, Carlos A Guzman and Claus-Michael lehr |
Topical application has always been considered an attractive option for drug administration; it seems to promise convenient, painless delivery of a range of drugs, either locally or systemically. But living up to this promise requires the drug formulation to traverse the dermis without disrupting the skin’s protective barrier function. This is a major challenge and has been the subject of intense research for many years. Now, fresh data suggest that nanotechnology-based formulations could exploit the hair follicle to transport drugs through the skin. Could this be the start of a new initiative in skin-mediated drug delivery?
Skin is the first line of defense against infectious agents, toxic substances and environmental stresses. Critical to this barrier function is the outermost “dead” layer of the skin – the stratum corneum, which is tough, hydrophobic and impermeable. These attributes present significant challenges from a drug delivery perspective. Nevertheless, interest in the skin as a route of drug administration remains high; advantages include accessibility, good patient acceptability, and avoidance of first-pass effects and other complications associated with the oral route. In addition, the skin itself may be a therapeutic target. Until recently, however, the transdermal route has appeared to be feasible only for a limited group of active substances with favorable properties of size and lipophilicity. This may now be changing, due to a better understanding of hair follicle-mediated drug delivery.
Gland designs
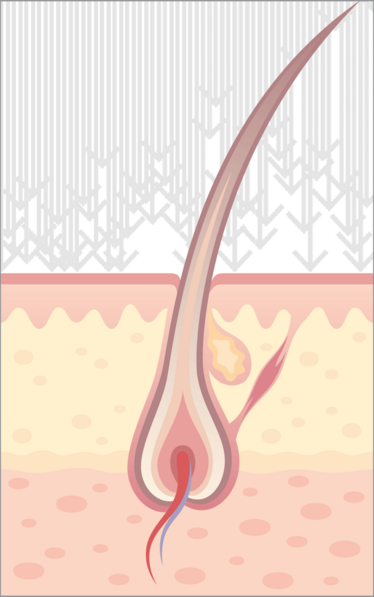
It has been known for many years that the pilosebaceous unit (consisting of the hair follicle and sebaceous gland – see Figure 1) can play a role in the passive transport of some drugs into the skin (1, 2). Nevertheless, to reach the epidermis and egress from the skin into circulation, the drug still must penetrate the keratinocyte layers surrounding the hair shaft. This fact, together with the rather low skin area occupied by hair follicles, has led to the assumption that this was a route of limited potential, and as such unworthy of further investigation (3). Therefore, various other strategies for penetrating the skin barrier have been adopted – and continue to be used; for example iontophoresis, microneedles, lasers and jet injectors. Recently, however, the follicular route has received renewed attention as a potential “bypass” option (4). Initial studies used liposomes as drug carriers, due to their chemical similarity with the secretions of the sebaceous gland (5, 6). Some gene therapy approaches have also focused on the hair follicle, due to its populations of resident stem cells and lineages of rapidly dividing cells (which are predisposed to take up and express exogenous DNA) (7, 8).
The most significant development has been the growing and pervasive impact of nanotechnology in cosmetics and drug delivery; in fact, this has now brought about a change of perspective in topical formulations. In particular, toxicological studies showed that particulate carrier systems accumulate in the hair follicle in a size-dependent manner (9). This observation was made when researchers analyzed sunscreens containing titanium dioxide (TiO2) microparticles (which help block UV radiation) (10). It was found that most of the TiO2 particles remain on the skin surface; however, a small amount accumulated in the hair follicles (10), which demonstrates the potential of the hair follicle to mediate drug perfusion in the skin.
Subsequent studies showed that particulate carrier systems not only permitted deeper penetration of marker substance into hair follicles than when the substance is in free form, but also facilitated a considerable depot effect (9). The follicular route has now been tested for its ability to mediate delivery of various substances, including those which would be expected to profit from the potential reservoir effect provided by follicular accumulation: hydrocortisone, interferon A, cyclosporine A, testosterone, estradiol and treatments for hair loss. Indeed, the follicular route may be particularly beneficial for hormone therapies, as hair follicles provide a means to achieve relatively deep penetration, and thus relatively large depots. Furthermore, careful design of the particulate carrier can enhance the depot effect, and also tailor the release profile of the drug (11). Finally, a particulate carrier system may be essential for skin-mediated delivery of certain drugs – in particular, to carry large and/or hydrophilic substances (that are unlikely to penetrate skin at all by conventional transdermal pathways) into the deeper skin layers.
Microorganisms are also able to use the follicular route, and consequently the immune system is well-represented and active in the skin. The accessibility of skin-resident immune cells, together with the potential for particulate carrier-mediated delivery of macromolecules into the skin via the hair follicle, suggests that the follicular route would be an effective administration option for vaccines. Early studies relied on pre-treatment – such as cyanoacrylate stripping of the skin surface – to facilitate vaccine penetration. This method of vaccination was highly efficient, since skin stripping both allows deeper penetration into skin and hair follicles, and also generates a stress stimulus that amplifies the resulting immune response (12, 13). However, this is still an invasive method and leaves room for improvement – particularly with regard to patient comfort and compliance. A simplified, non-invasive method, involving massage of substances into the skin, has since presented itself as a strategy for achieving permeation into the hair follicles without pre-treatment. For example, Baleeiero and colleagues applied antigen-loaded SiO2 microparticles to the skin prior to massage, and observed that such particles penetrated into the follicle and delivered the antigen to perifollicular antigen presenting cells (14).
Reaching the root
Inspired by such data, we have developed biodegradable and biocompatible polymeric carriers consisting of poly(lactic-co-glycolic acid) (PLGA) nanoparticles, with or without an outer coating of the equally biocompatible polymer chitosan. By focusing on biopolymers, we aim to avoid any adverse effects related to the formulation. We also chose to pursue a non-invasive approach to carrier system administration, without application of any pre-treatment measures. In addition, we investigated the effect of carrier surface properties (charge and hydrophobicity) on hair follicle penetration (15).
Briefly, we developed a biopolymer formulation of the model antigen ovalbumin, in combination with the adjuvant bis-(3’,5’)-cyclic dimeric adenosine monophosphate. After non-invasive, topical administration to the skin of mice, our system elicited efficient antigen-specific humoral and cellular immune responses to ovalbumin. Furthermore, the barrier function of the skin of the mice remained intact (16), (17). Optimization of the carrier system to permit higher protein loading enabled generation of an immune response after only two booster treatments (17), (18).
Despite the promising results, however, it’s important to remember that skin-targeted particulate carrier systems still require development; for example, to allow targeting to a specific type of hair follicle or specific cell types (in particular, antigen presenting cells), to facilitate more efficient combinations of antigen(s) and adjuvant, or to reduce the need for boosting. That said, we firmly believe that the hair follicle represents a promising drug administration route, with potential advantages including:
- ability to access specialized cell populations like stem cells and immune cells
- potential to act as a depot
- non-invasive and painless
- may be amenable to self-administration, rather than demanding application by expert personnel.
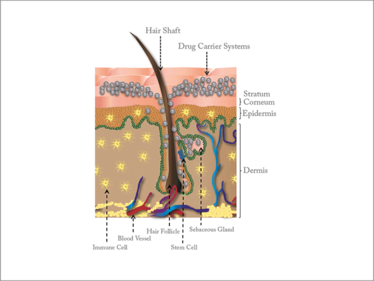
Figure 1. Cross-section of the skin, showing various structures and cell populations accessible to drugs delivered via the hair follicle. Drugs and drug carrier systems can penetrate into the hair follicle and be stored as depots within the sebaceous gland; interaction with immune cells (vaccination) and stem cells (regenerative medicine) is also possible.
The follicular route is not a dead-end street. Rather, for particulate carrier systems of carefully tailored and optimized design – in which nanotechnology will be key – the hair follicle may provide a well-connected access point for a broad spectrum of new applications, in vaccines, and in a broad range of therapeutic fields, such as allergy, skin disorders and regenerative medicine (for example, hair-loss therapy). We encourage anyone interested in our work, or in the field of follicular drug delivery in general, to delve into the references for further information.
- B Illel, H Schaefer, “Transfollicular percutaneous absorption. Skin model for quantitative studies”, Acta Dermato-Venereologica, 68, 427–430(1988).
- H Schaefer et al., “Follicular penetration”, In: RC Scott, RH Gu, I Hadgraft (Eds), Prediction of Percutaneous Penetration: Methods, Measurements, and Modelling, IBC Technical Services, London, 163–173 (1990).
- RJ Scheuplein, “Mechanism of percutaneous absorption”, J Invest Dermatol, 48, 79–88 (1967).
- J Lademann et al., “Hair follicles as a target structure for nanoparticles”, J Innov Opt Health Sci, 08, 1530004 (2015).
- LM Lieb, et al “Topical delivery enhancement with multilamellar liposomes into pilosebaceous units: I. In vitro evaluation using fluorescent techniques with the hamster ear model”, J Invest Dermatol, 99, 108–113 (1992).
- L Li et al., “Product-delivering liposomes specifically target hair follicles in histocultured intact skin”, In Vitro Cell Develop Biol - Anim, 28 A, 679–681 (1992).
- L Li, RM Hoffman, “The feasibility of targeted selective gene therapy of the hair follicle”, Nat Med, 1, 705–706 (1995).
- A Domashenko, S Gupta, GC Cotsarelis, “Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex”, Nat Biotech, 18, 420–423 (2000).
- J Lademann et al., “Nanoparticles – an efficient carrier for drug delivery into the hair follicles”, Eur J Pharm Biopharm, 66, 159–164 (2007).
- J Lademann et al, “Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice”, Skin Pharmacol App Skin Phys, 12, 247–256 (1999).
- WC Mak et al., “Triggering of drug release of particles in hair follicles”, J Control Release, 160, 509–514 (2012).
- R Toll et al., “Penetration profile of microspheres in follicular targeting of terminal hair follicles”, J Invest Dermatol, 123, 168–176 (2004).
- A Vogt et al., “40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin”, J Invest Dermatol, 126, 1316–1322 (2006).
- RB Baleeiro et al., “Topical vaccination with functionalized particles targeting dendritic cells”, J Invest Dermatol, 133, 1933–1941 (2013).
- AS Raber et al., “Quantification of nanoparticle uptake into hair follicles in pig ear and human forearm”, J Control Release, 179, 25–32 (2014).
- A Mittal et al., “Non-invasive delivery of nanoparticles to hair follicles: A perspective for transcutaneous immunization”, Vaccine, 31, 3442–3451 (2013).
- A Mittal et al., “Efficient nanoparticle-mediated needle-free transcutaneous vaccination via hair follicles requires adjuvantation”, Nanomed Nanotech Biol Med, 11, 147–154 (2015).
- A Mittal et al., “Inverse micellar sugar glass (IMSG) nanoparticles for transfollicular vaccination”, J Control Release, 206, 140–152 (2015).
Hanzey Yasar is a PhD student, Sarah Gorden is Post Doc and Brigitta Loretz is a Scientist at the Department of Drug Delivery, Helmholtz-Institute for Pharmaceutical Research Saarland (HIPS), Helmholtz Centre for Infection Research (HZI), Saarbrücken, Germany.
Kai Schulze is a Scientist and Carlos Guzman is Professor and Head, Department of Vaccinology and Applied Microbiology, Helmholtz Centre for Infection Research (HZI), Braunschweig, Germany.
Claus-Michael Lehr is Professor, Department of Pharmacy, Saarland University, as well as co-founder and head of the Department of Drug Delivery, Helmholtz-Institute for Pharmaceutical Research Saarland.


















