Patient-Centric Printing
A proof-of-concept study indicates that stencil printing can be used to manufacture a range of flexible dosage forms

This article is part of our special focus on "traditional" pharma: The Small Molecule Manufacturer (read more here). You can find more articles from The Small Manufacturer here.

Henrika Wickström, Researcher, Åbo Akademi University, Finland.
In recent years, additive approaches to the manufacture of medicines have become more popular, offering companies the opportunity to develop flexible and tailored dosage forms that better meet patient needs. And while 3D and 4D printing are exciting avenues for the industry to further explore, stencil printing has thus far been overlooked.
Stencil printing refers to a low-cost, high-throughput printing technique that is used across various industries to produce everything from electronics to artwork – but it has not yet found its way into the pharma industry. In stencil printing, ink is transferred onto a substrate through a series of apertures in a printing plate. And, just like the stencils used by kids, the apertures determine the pattern and design of the printed product.
The test case
With an increasing focus on patient-centricity, companies must consider the best dosage form for the target population. When it comes to patients who struggle to swallow regular tablets, such as children and the elderly, orodispersible films represent an interesting option for the administration of low dose drugs – and a good test case for stencil printing. In a proof-of-concept study, my colleagues and I explored whether stencil printing could be used to manufacture the antipsychotic haloperidol in an orodispersible disc formulation suitable for children aged between 6 and 17. We also investigated whether the printing technique would lend itself to a decentralized manufacturing model that enabled production of medicines in close proximity to patients. Pharmacies and hospitals, in particular, would benefit from this approach as medicine could printed as and when needed – addressing potential deficits in product supply and giving them capacity to tailor doses to patients. Our findings supported this idea.
First, we designed a prototype flatbed printer that allowed us to produce orodispersible discs in a batch-wise manner. And then we turned our attention to the development of the API-containing ink – a significant hurdle for manufacturers looking into additive technologies. Indeed, we spent considerable time determining how the physicochemical properties of the API and its dispersion through the ink affected viscosity and therefore printing performance. Throughout the ink development process, we had to conduct a series of tests to assess i) the viscosity of the ink, ii) how well the liquid portion of the ink could adhere to the substrate used, iii) the removal of the dried film from the substrate and iv) if the binders and solvents would allow the ink to dry quickly enough to prevent delays to the overall production process.
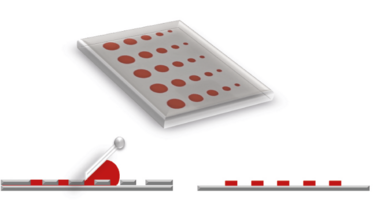
Figure 1. Stencil printing plate (top) and print result (right) of a flatbed process (left)
Success?
We were able to print our haloperidol-containing ink to produce final dosage forms that varied in strength between 0.5 mg and 2.5 mg of API, making them suitable for the treatment of children across the target age range. And, I must admit, we were excited by the results – they proved to us that stencil printing has the potential to be used as a pharmaceutical manufacturing technique. At the same time, we know we have to maintain a sense of realism about the technology. Though we were able to create a range of flexible dose forms with our stencil printer, the formulation of inks containing other relevant APIs is still a challenge.
We also acknowledge that we could optimize our drying times by adjusting the solvent composition and amount. It would also be interesting to study crystalline, pasty inks for stencil printing to evaluate the printability of another type of ink formulation.
The future
In short, our study established that stencil printing can be used successfully to manufacture orodispersible medicines, and we’ve already identified some technical areas for improvement. Next, we – and the wider pharmaceutical community – must answer additional questions to ensure the suitability and viability of stencil printing in commercial production; in particular, how can this technology be adapted to meet GMP requirements? Is it possible to develop an automated stencil printer with its own drug-containing ink supply? Can stencils be reused without compromising the integrity of drug products?
As with any technology, we must remember that different additive manufacturing techniques have unique strengths and weaknesses; though stencil printing was shown to be effective in this particular instance, another technology may be better suited to the development of other dosage forms. In-line quality control methods will need to be developed to ensure the quality of stencil printed products at various dose strengths. It will also be important to assess the benefit to patients versus the cost of production. I think it will be interesting observe the interplay between health professionals and those who develop specialist printing technologies over the coming years.
But in my opinion, there is a place in the pharmaceutical industry for a variety of additive manufacturing technologies, including stencil printing. They work well alongside more conventional manufacturing techniques, allowing companies to flexibly produce more niche products. With a broader range of manufacturing options, companies will be better equipped for their pursuit of patient-centric drug formulations.