Introduction
According to the definition established by the FDA, a generic drug is "a drug product which is comparable to a reference listed drug (RLD) product in dosage form, strength, route of administration, quality, performance characteristics, and intended use". Rigorous rules and regulations pertaining to abbreviated new drug application (ANDA) submissions are complex and the generic drug industry strives to meet these regulations to obtain FDA's approval. And being the "first to file" is the most fundamental principle in the generics business because several companies compete to create generics of successful products going off patent. Therefore, generics companies must be highly skilled and disciplined in product development and achieving bioequivalence-the most critical development area. Though generics companies commonly use reverse engineering techniques, the topic and, more importantly, the tools needed to carry out the process are rarely discussed in the public domain. In this application note we discuss one such tool that can be used for oral solid dose formulations.Case Study
Two cold remedy formulations were analysed on the Morphologi G3-ID: one was a commercial brand and the other a generic one. Individual components within the formulations were identified by comparing their Raman spectra with those in a commercial database. Once the components were identified the particle size distributions of individual components in each formulation were compared, as was the overall composition of the two formulations.Methodology
The cold remedies were dry powder formulations that were automatically dispersed and analysed using the Morphologi G3 -ID. 13 mm3 of sample was dispersed using the instrument's integrated dry powder dispersion unit using the low pressure dispersion option. Figure 1 shows an example image of one of the dispersed samples. The samples were morphologically analysed using the 5 x objective. Particles with a circular equivalent diameter (CED) larger than 25 µm were targeted for the Raman chemical identification. In this case the acquisition time was 10 seconds for each particle and spectra from a few thousand particles from each sample were gathered in an overnight analysis.
Figure 1: Example image of dispersed sample (5x magnification).
Results and discussion
Figure 2 presents the overlays of the Circular Equivalent Diameter (CED) particle size distributions of the two cold remedies by number and volume respectively. The commercial brand contains more particles in the 30 to 200 µm range than the generic brand. The number based distribution indicates the commercial brand also contains more fine particles less than 20 µm in size.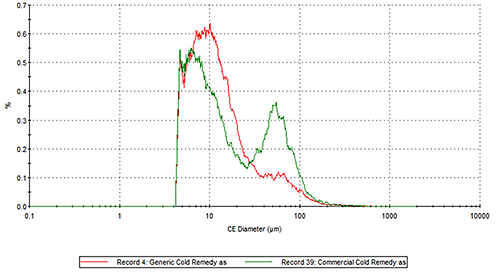
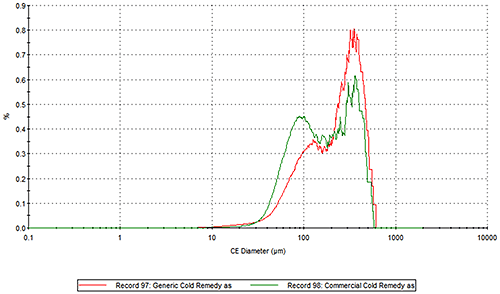
Figure 2: Overlay of the number based (top) and volume based (bottom) CED distributions
Component identification
Both of the cold remedies are made of more than ten components, including the two active pharmaceutical ingredients (APIs) Paracetamol and Phenylephrine. Since pure materials were not available from which to create reference libraries, Raman spectra acquired by the Morphologi G3-ID from 11 different components were identified and these were used as reference library spectra. As examples, the reference spectra for the components 1, 2 and 11 are displayed in figure 3.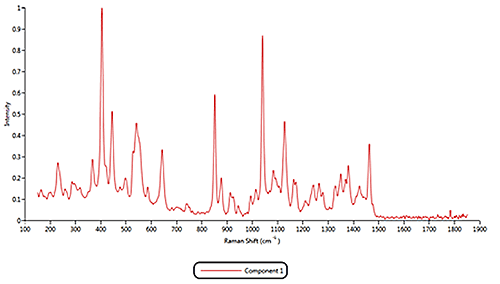
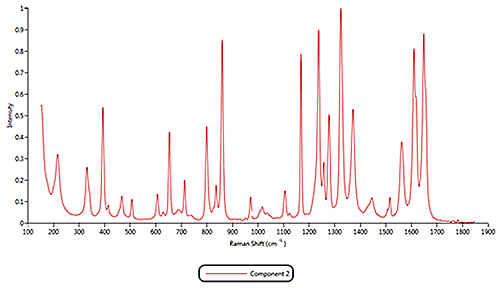
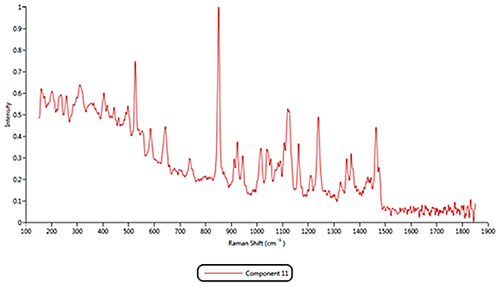
Figure 3: Raman spectra collected for components 1, 2 and 11 Based on the reference spectra, it is possible to classify individual particles as being one of the formulation components. During this classification process a correlation calculation is performed between each particle's spectrum and the reference spectra and a correlation score generated. A score close to 1 indicates a good match whereas a score close to zero indicates no match. Chemical classifications based on these correlation scores were applied to the results to identify the proportion of each component present in the two formulations. The results of the volume based classifications and are presented in figure 4. The three most common components in both formulations were components 1, 2 and 11. The commercial product contains more of component 1 than the generic product whereas the generic product was found to contain more of component 2. >> Free download of the full Application Note as PDF
Malvern Instruments provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations and our commitment to maximizing the potential of established techniques. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. Headquartered in Malvern, UK, Malvern Instruments has subsidiary organizations in all major European markets, North America, Mexico, China, Japan and Korea, a joint venture in India, a global distributor network and applications laboratories around the world. www.malvern.com severine.michel@malvern.com





