
RNA therapeutics are a rapidly evolving class of drugs that harness the ability of RNA molecules to modulate gene expression, offering novel strategies for addressing disease mechanisms at the genetic level. While traditional drug development is centered around small molecules or protein therapies, these products target a marginal proportion of the human genome (0.05%) and require the presence and identification of active sites for small molecule binding (1). RNA-based therapies utilize various RNA species to exert unique functions on genes, transcripts, and proteins. These therapies offer distinct advantages, including high specificity and the ability to pursue previously “undruggable” targets.
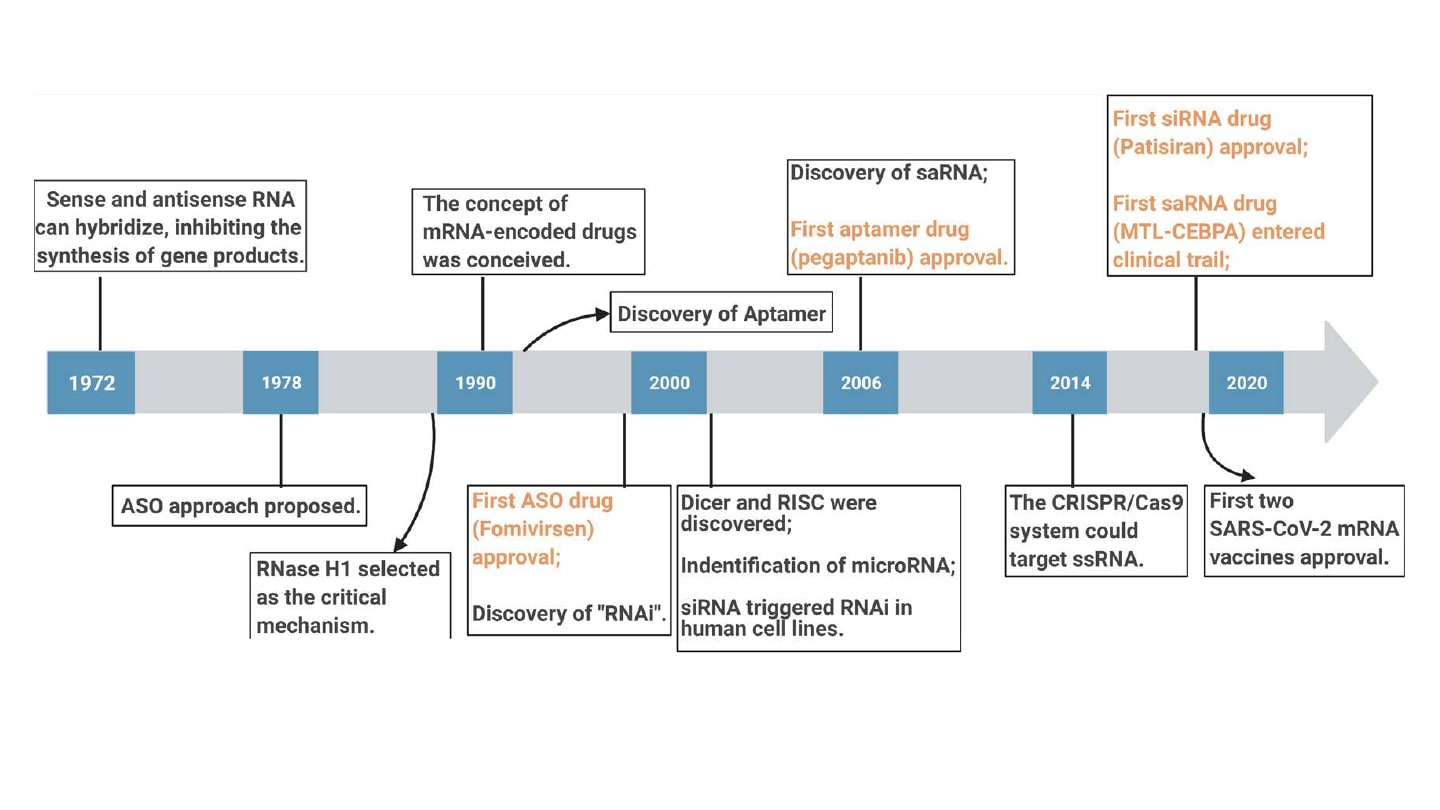
Initial interest in and the advancement of RNA-based approaches was enabled by the critical observations that antisense oligonucleotides (ASOs) could inhibit translation and replication of Rous sarcoma virus and that small, double-stranded RNAs could selectively silence gene expression in Caenorhabditis elegans and mammalian cells (2-5). Since these discoveries, RNA interference (RNAi) has become a foundational tool for developing RNA therapeutics.
More recently, preclinical and clinical studies of in vitro transcribed mRNA have bolstered the development of mRNA vaccines for applications such as cancer immunotherapy and infectious diseases (6). Further, the COVID-19 pandemic demonstrated mRNA-based therapies’ rapid production, scalability, and cost-effectiveness. With numerous drugs employing RNA technologies that have received approval from the FDA, RNA therapeutics are redefining the treatment landscape for genetic, infectious, and chronic diseases. However, due to the precise and sensitive nature of therapies involving RNA, several considerations associated with their development and production are necessary to ensure efficacy and safety.
The basics of RNA development and production
The development of therapies containing RNA requires nucleic acid synthesis and the use of stabilization methods to promote their therapeutic utility in vivo. Advances in RNA production techniques, including chemical synthesis, enzymatic transcription, and bioproduction using engineered microbes, have enabled the scalable manufacturing of therapeutic RNA. RNA molecules can be synthesized through various nucleic acid production methodologies, including solid-phase chemical synthesis and DNA template-dependent in vitro transcription using bacteriophage-derived RNA polymerases (7). High-yield, large-scale RNA bioproduction capabilities have also increased via the use of RNase III-deficient bacterial strains or specialized RNA carrier systems to facilitate the generation of noncoding RNAs. Several modifications and optimization protocols have also been adopted to improve the stability and expression of synthetic mRNAs (6). These include techniques such as screening and selecting the 5’ and 3’ untranslated regions to ensure translation efficiency, codon optimization, and nucleoside modifications or sequence engineering to fine-tune immunogenicity or prevent mRNA decay.
To overcome RNA’s intrinsic instability and its susceptibility to degradation, various strategies and delivery systems involving liposomes, polymer nanomaterials, or nanoparticles have been developed to protect the sequence and facilitate its transport into cells. Cationic lipid-based liposomes are frequently employed to encapsulate RNA molecules, providing structural protection and promoting cellular uptake through a composition akin to the cellular membrane. Additional delivery modalities leverage polymeric nanocarriers and inorganic nanoparticles, such as those composed of silica, carbon, or gold, to stabilize the RNA and facilitate intracellular transport.
Challenges and regulatory considerations
Advancements in RNA synthesis, stabilization, and delivery technologies have significantly enhanced the feasibility of commercially developing and manufacturing RNA-based therapeutics. Despite these successes, numerous challenges persist in the areas of target identification and validation, large-scale production, and quality control (7).
Uncovering and attributing certain RNA species and their functions to specific disease phenotypes requires thorough mechanistic studies employing both in vitro cell culture systems as well as in vivo animal models. This target identification and biological validation process is imperative for the rational design of RNA therapeutics, although it remains highly disease-specific and experimentally intensive. In addition, beyond molecular target selection and validation, the optimization of RNA chemical structure is critical during the development phase. Post-synthesis chemical modifications of RNA are essential for optimizing its stability, pharmacokinetics, and translational efficiency. For example, substituting uridine residues with synthetic analogs has been demonstrated to enhance translation efficiency. Additionally, the incorporation of polyadenylated (poly-A) tails onto synthetic mRNA molecules has been shown to increase their stability, extend their half-life, and improve their processing in cells.
Following target selection, validation, and molecular optimization, RNA production at the commercial scale presents several technical hurdles. The manufacturing process must be scaled to yield sufficient quantities of RNA for clinical evaluation and subsequent commercial distribution. This scale-up process often necessitates substantial modifications, particularly in downstream purification steps, to maintain product that is similar in yield and quality to that produced at laboratory scale. Determining product purity is another critical aspect of RNA therapeutic manufacturing to ensure its efficacy and safety. A comprehensive strategy must be implemented to detect and eliminate impurities such as the byproducts of manufacturing (e.g., unincorporated nucleosides, truncated or abortive RNA transcripts, template DNA) and microbial contaminants.
RNA-based therapies also pose unique regulatory considerations. The COVID-19 pandemic drove the accelerated development and approval of mRNA vaccines, which received conditional marketing and emergency use authorizations in Europe and the US, respectively. Consequently, the regulatory framework has not kept pace with the speed of mRNA therapeutic development and production, although it will continue to evolve. To date, preclinical studies of RNA therapeutics have relied on traditional analytical techniques, such as quantitative whole-body autoradiography (QWBA) and mass spectrometry-based assays, to obtain biodistribution data and meet specific regulatory requirements. However, these techniques have limitations. For example, QWBA fails to distinguish a drug from its metabolites or degradation products, which may be an issue given the instability of RNA molecules. Further, in mass spec-based assays, properties such as high molecular weights, degree of anionicity, and instability of phosphate linkages raises the detection limits for RNA compared with small molecules. Thus, additional techniques that offer improved precision and sensitivity should be considered, as they may support in vivo characterization and detection of low-abundance targets.
Analytical solutions
Although the development of RNA therapeutics presents various technical and regulatory hurdles, the implementation of robust analytical methodologies early during the process increases the likelihood of achieving consistent manufacturing outcomes while minimizing potential impurities or contaminants. Commonly used techniques generally fall into proteomic and genomic categories. Proteomic assays, such as enzyme-linked immunosorbent assays, flow cytometry, or immunohistochemistry, serve as indirect functional readouts of RNA activity by measuring translated protein products and their downstream biological effects. However, while they are valuable for assessing therapeutic efficacy, proteomic techniques provide limited information regarding the physicochemical properties or structural integrity of the RNA molecule itself. Meanwhile, genomic assays are specifically designed to detect and quantify nucleic acids, enabling the direct analysis of RNA species as well as their intracellular transcriptional activity. Many techniques, including in situ hybridization, liquid chromatography with ultraviolet detection (LC-UV), and liquid chromatography with tandem mass spectrometry (LC-MS/MS), are well-developed, but each comes with a set of limitations. Hybridization is dependent upon probe specificity and sensitivity as well as detection chemistry, which can affect the background signal. LC-UV and LC-MS/MS offer high specificity, and the latter has a broad dynamic range, but both methods require extensive sample preparation. Further, LC-MS/MS may exhibit reduced sensitivity for certain RNA analytes.
Reverse transcription polymerase chain reaction (RT-PCR) is a keystone technique that offers high specificity and sensitivity for the quantification of target RNA molecules. The quantitative robustness of RT-PCR is largely dictated by the specific method that is used. Real-time quantitative PCR (qPCR) relies on the generation of a standard curve, the accuracy of which may be influenced by low target abundance. This requirement as well as variable amplification efficiency may impact development timelines and data reliability. In contrast, Droplet Digital PCR (ddPCR) enables absolute quantification of RNA molecules with superior precision, even in scenarios involving low template copy numbers. By partitioning samples into droplets that undergo individual PCR reactions and applying Poisson statistics, the need for extrapolation from a standard curve is eliminated (8). Additionally, ddPCR method allows for rapid, high-throughput analysis and requires low sample input. Because precise and accurate target quantification is imperative to ensure the potency, dosage, quality, and purity of RNA-based therapies, ddPCR technology offers numerous advantages to support their development, production, and testing (Figure 2). It can be used as an analytical tool across these stages, with the ability to address needs such as therapeutic target identification, dosage quantification, ratio determination for multivalent therapies, contaminant detection, and evaluation of gene expression before and after treatment.
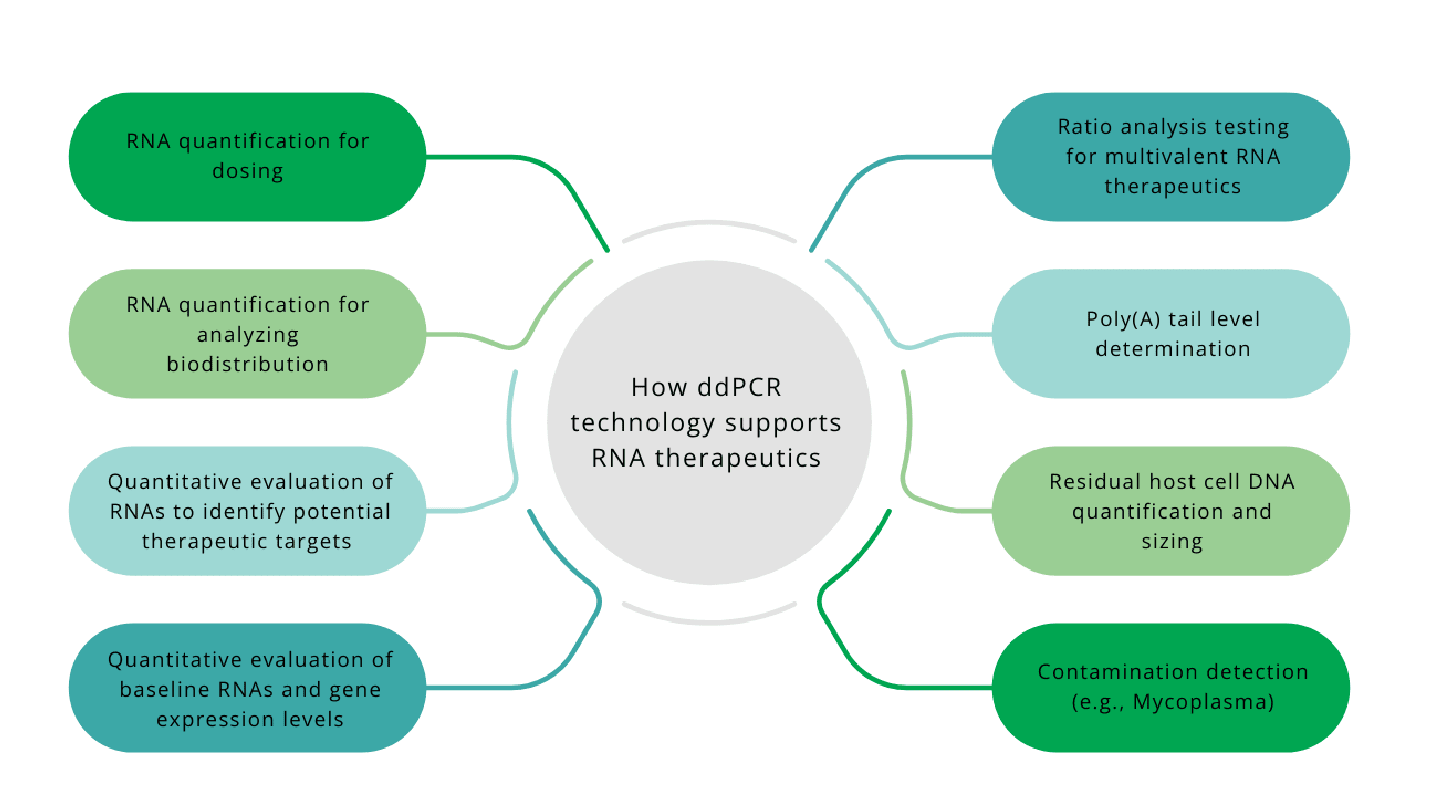
Applications of ddPCR technology in RNA
The utility of ddPCR technology is evident across a range of RNA therapeutic applications, where accurate and sensitive quantification of RNA molecules is essential (8). This includes mRNA-based therapies, as in the case of vaccines developed against COVID-19, and other RNA modalities like ASOs used in the treatment of genetic disorders. ddPCR technology has been validated as a highly sensitive method for the detection of SARS-CoV-2, the virus that causes COVID-19, including in clinical samples with low viral RNA concentrations (9). In addition to its diagnostic applications, ddPCR method was employed for the assessment of poly-A tail integrity during the development and quality control of the Pfizer-BioNTech mRNA-based COVID-19 vaccine (10). The ability to accurately quantify such sequence-specific features highlights ddPCR's solutions utility in the analytical characterization of mRNA therapeutics.
Beyond mRNA vaccines, ddPCR technology has been used in the research and development of ASO therapies for neuromuscular disorders such as muscular dystrophies (11). Duchenne muscular dystrophy (DMD), the most common and severe subtype, results from genetic mutations that impair the production of dystrophin, a structural protein whose absence leads to progressive muscle deterioration. One therapeutic strategy for DMD involves the use of ASOs to promote exon skipping within the dystrophin pre-mRNA, thereby restoring the reading frame and enabling mRNA expression. ddPCR method provides absolute quantification of both therapeutic oligonucleotides and the transcripts that are expressed, enabling robust assessment of treatment efficacy.
In conclusion, RNA therapeutics represent a promising and rapidly advancing field with transformative potential in treating a wide range of diseases. However, the path to their production at the commercial scale is complex and requires the availability of reliable analytical tools. ddPCR technology has emerged as a key analytical technology that offers numerous advantages in overcoming the challenges associated with the development and quality control of RNA-based therapies due to its high sensitivity, specificity, and capacity for absolute quantification of target molecules. The successful clinical applications of ddPCR solutions, particularly in the accelerated production timelines of COVID-19 mRNA vaccines, emphasize its critical contribution to ensuring the efficacy and safety of these novel treatments.
Teaser Image Credit: Adobestock.com
References
- Y Zhu et al., “RNA-based therapeutics: an overview and prospectus,” Cell Death Dis. (2022). doi: 10.1038/s41419-022-05075-2
- SM Elbashir et al., “Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells,” Nature (2001). doi: 10.1038/35078107
- A Fire et al., “Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans,” Nature (1998). doi: 10.1038/35888
- ML Stephenson, PC Zamecnik, “Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide,” Proc Natl Acad Sci U S A (1978). doi: 10.1073/pnas.75.1.285
- PC Zamecnik, ML Stephenson, “Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide,” Proc Natl Acad Sci U S A (1978). doi: 10.1073/pnas.75.1.280
- H Parhiz, EN Atochina-Vasserman, D Weissman, “mRNA-based therapeutics: looking beyond COVID-19 vaccines,” Lancet (2024). doi: 10.1016/S0140-6736(23)02444-3
- Bio-Rad, Bulletin 3506. Available at: https://www.bio-rad.com/sites/default/files/2024-06/3506.pdf
- Bio-Rad, Bulletin 3456. Available at: https://www.bio-rad.com/webroot/web/pdf/lsr/gated/Bulletin_3456.pdf
- A Ishak A et al., “Diagnostic, Prognostic, and Therapeutic Value of Droplet Digital PCR (ddPCR) in COVID-19 Patients: A Systematic Review,” J Clin Med (2021). doi: 10.3390/jcm10235712
- EMA, Assessment report: Comirnary, COVID-19 mRNA vaccine (nucleoside-modified). Available at: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf
- A Aartsma-Rus, GJ van Ommen, “Antisense-mediated exon skipping: a versatile tool with therapeutic and research applications,” RNA (2007). doi: 10.1261/rna.653607




