Welcome to our annual showcase of top technology launches this year for drug development and manufacturing! Every year, The Medicine Maker asks readers to submit their nominations for inspiring technologies that have recently launched.
This showcase has been compiled based on those nominations. Key trends include solutions for accelerating drug development and improving efficiency. In today’s competitive market, speed is everything.
cartriQ 5 ml sterile cartridges (SCHOTT Pharma)
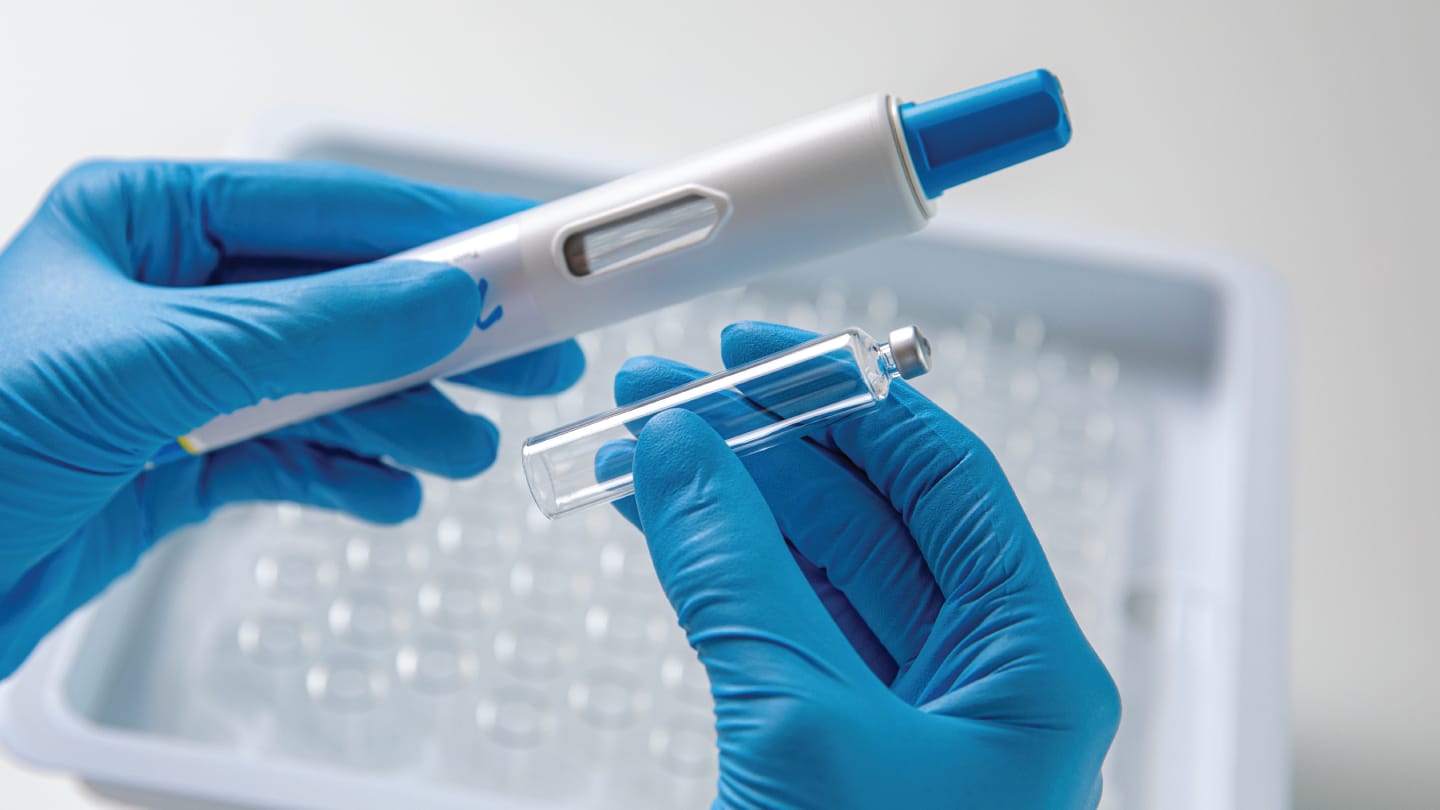
Large-volume glass cartridges for autoinjectors
Until recently, the choice of containers for self-administration devices handling volumes above 3 ml was limited. While conventional prefilled syringe autoinjectors were available in volumes of up to 2.25 ml, cartridge-based autoinjectors were available in volumes of up to 3 ml. For higher volumes, on-body injectors represent the next established step.
SCHOTT Pharma’s cartriQ 5 ml ready-to-use (RTU) cartridge enables high-volume, high-viscosity formulations to be administered via autoinjectors and pen injectors, bridging clinical-grade performance with at-home usability. Co-designed with SHL Medical to ensure seamless integation with Maggie 5.0 as well as patient-friendly usability, it features baked-on siliconization and ISO-compliant geometry for drug stability and reliable fill-and-finish integration.
CRA Agent (Medable)

An agentic AI solution that proactively automates and optimizes clinical trial monitoring
According to early research by Medable, over 60% of a clinical research associate’s time is spent on administrative tasks. The CRA Agent connects data across more than a dozen systems, monitors hundreds of trial variables in real time, and recommends next-best actions. By automating routine tasks with oversight, it frees CRAs to focus on what only humans can do: build trust with sites, support patients, and apply clinical judgment. Further, it allows ‘human-in-the-loop’ collaboration to help ensure proper oversight to help ensure patient safety.
CRA Agent is offered on Medable’s Agent Studio – a no-code agent builder that enables clinical teams to quickly configure AI agents for various use cases. The platform has been shaped by insights from researchers, clinical trial operators, and regulators on the front lines of clinical development.
Design2Optimize (Lonza)

A digital twin-driven platform that streamlines small molecule API development via smart, optimized experimentation
By integrating model-based design of experiments, digital twin simulations, and advanced multi-criterial optimization, Design2Optimize aims to enable faster, smarter, and more cost-effective development. The platform identifies the most valuable experiments, builds predictive models, and runs thousands of in silico simulations in seconds. This approach dramatically reduces lab time and resource use, enabling researchers to make faster decisions and unlock innovations earlier in the process. Its optimization engine ensures processes are fine-tuned for yield, throughput, and efficiency. According to Lonza, the platform can ensure optimal processes and faster clinical readiness. By minimizing physical experimentation and maximizing digital insight, it accelerates innovation (especially in early-phase, highly disruptive and innovative projects) and enhances scalability.
Domina (IMA S.p.A)

DOMINA is a modular technological platform for Pharma 4.0 manufacturing excellence
This tablet press is designed as a modular technological platform that allows pharmaceutical manufacturers to configure the ideal setup for any powder and tablet type. Its plug-and-play architecture allows seamless transitions between mono and bilayer production, even with hard-to-compress powders. It features a patented ‘Dynamicam’ filling system, feeding solutions with single-motorized paddles, and Preforma, a special compaction cam that can make the difference in enhancing powder de-aeration, reducing the risk for lamination and capping. It also integrates Pharma 4.0 principles with advanced automation, adaptive diagnostics, and the Kortex MAX HMI system, ensuring data integrity, cybersecurity, and predictive analytics. Domina’s adaptability to different formulations and production needs allows manufacturers to respond quickly to market demands.
iDFill (BD)

RFID-based solution for prefillable syringes designed to improve traceability during fill-finish process and distribution
Drug mix ups are expensive and color-coded product identification has limitations in accuracy and scalability. To address this, BD has developed prefillable syringes pre-tagged with unique serial numbers referred to as the Container Unique Identifier (CUID). This innovation enables unit-level traceability throughout the product lifecycle, aiming at supporting compliance and manufacturing excellence.
The CUID can be scanned at various stages of the manufacturing process, from filling to secondary packaging including assembly. The approach is designed to support continuous verification and data integrity across the production workflow. The syringe external dimensions remain unchanged to ensure compatibility with secondary devices and the barrel also remains clear for visual inspection. Beyond traceability, the solution has the potential to create a digital backbone that unifies process and equipment data around the CUID. This integrated dataset would enable advanced analytics and AI applications such as root cause analysis, predictive quality control, and parametric release, transforming fragmented workflows into connected manufacturing ecosystem. CDMO ten23 health has been involved in piloting the solution.
Immucise (Terumo Pharmaceutical Solutions)

Intradermal injection system designed to deliver vaccines and other approved drugs to the dermal layer of the skin
The dermis is extremely rich in various resident and recruited types of dendritic cells, which play a critical role in the human immune response by capturing antigens and presenting them to T cells. Intradermal administration of vaccines can potentially result in quantitatively or qualitatively superior immune responses compared to intramuscular or subcutaneous injection (1).
The Immucise intradermal injection system is designed to be simple enough to handle easily and is expected to reduce risks of damaging tissues, such as blood vessels and peripheral nerves, due to its thin and short needle (2). In an ‘FDA guidance based Human Factors Engineering study’ on the Immucise Intradermal Injection System, conducted by Terumo, a group of healthcare professionals (55 participants including physicians, primary care nurses, and pharmacists) were able to complete an injection using the Immucise Intradermal Injection System without use error on critical task, and without receiving any prior instructions on the system (3).
References: 1 PATH, WHO, “Intradermal Delivery of Vaccines. A review of the literature and the potential for development for use in low- and middle income countries” (August, 2009), 2 R Arakane et. al., “Immunogenicity and safety of the new intradermal influenza vaccine in adults and elderly: A randomized phase 1/2 clinical trial,” Vaccine, 33, 6340-6350 (2015), 3 Immucise Intradermal Injection System Human Factors and Usability Engineering Study Report (sponsored)
MEDELLAPRO (Gelita)

An ultra-low endotoxin gelatin
MEDELLAPRO is part of Gelita’s endotoxin-controlled excipient portfolio. It is a purified gelatin that features ultra-low levels of endotoxins (reaching <= 10 endotoxin units/g), making it suitable for high-standard applications in bioscience development where gelling properties and purity are key, such as 3D bioprinting, scaffolds, cell culturing, and more. As a natural biomaterial, it is well tolerated by mammalian cells and human tissue. Gelita’s endotoxin removal process was developed efficiently over approximately two and a half years, culminating in the submission of a patent application. The company claims that this is the first ultra-low endotoxin gelatin in the market that uses food-grade phospholipids for endotoxin depletion without cytotoxic substances.
NK.SET Synthetic Promoter Library (SynGenSys)
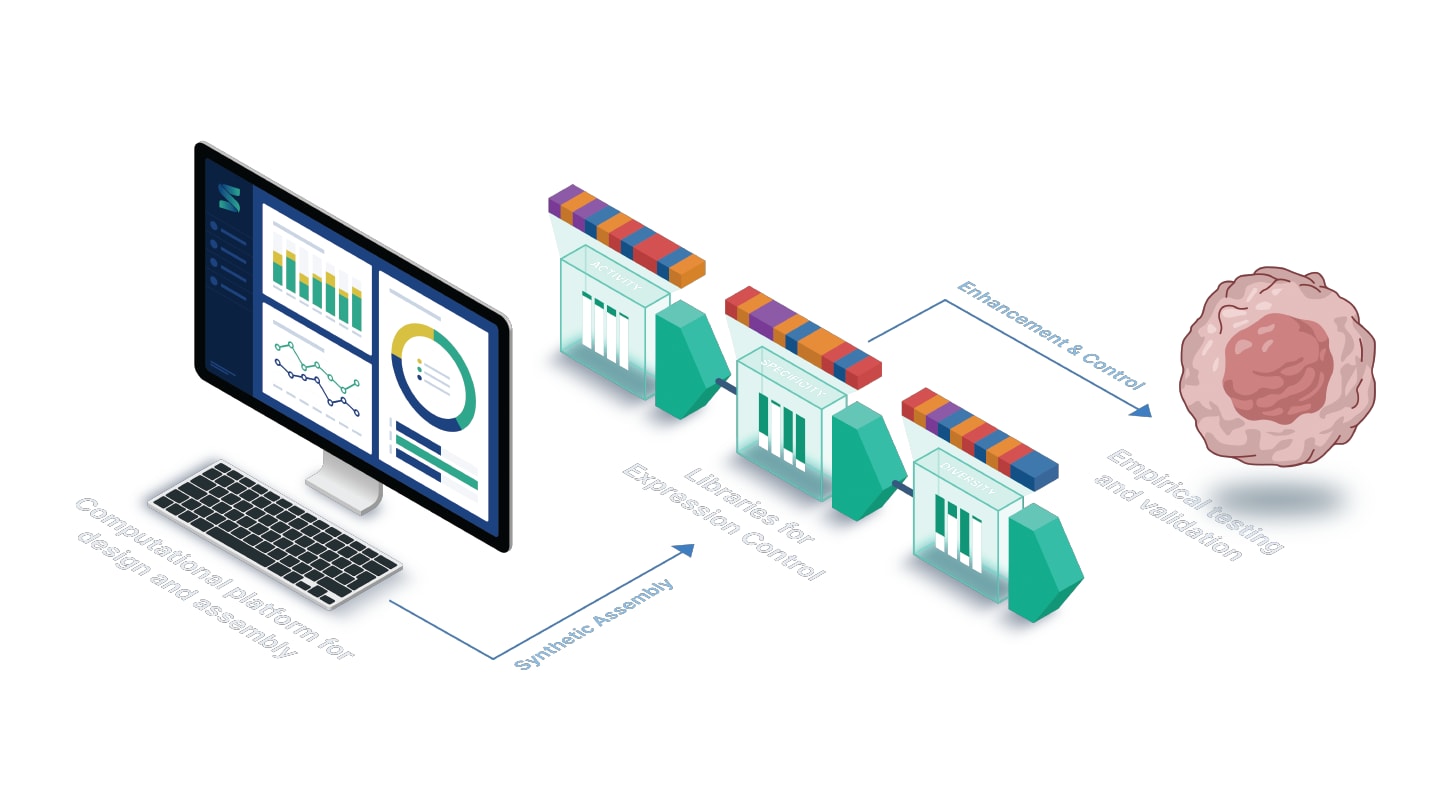
Synthetic promoters that enable tuneable gene expression for NK cell immunotherapies
Promoter selection is a critical yet often overlooked step in cell and gene therapy development, with many manufacturers opting for natural promoters that come with fixed sizes, activity profiles, and regulatory behaviors, thus hindering development by limiting access to precise, cell-specific, tuneable transgene expression. NK.SET synthetic promoter library enables cell-specific control over gene expression. The promoters have a range of compact de novo sequences of ~200-600 bp with customizable expression levels. Each promoter is rationally designed to reduce off-target activity and can be tailored to meet specific therapeutic needs. Moreover, the minimal promoter size increases vector therapeutic payload capacity, supporting application across diverse therapeutic contexts.
Nuvia wPrime 2A Media (Bio-Rad Laboratories)

Tunable, scalable weak anion exchange hydrophobic interaction mixed-mode chromatography resin
Nuvia wPrime 2A Media helps to enhance the efficiency, yield, and economics of biopharmaceutical manufacturing. The resin’s tunable binding and milder elution profiles help preserve target molecule stability, reduce aggregation, and enable processing therapies that might otherwise be lost or damaged using harsher chromatographic conditions.
As a mixed-mode chromatography resin, the resin supports process intensification, reducing the number of chromatography steps, material, labor, and operating costs. Unlike strong AEX and AEX-HIC resins that remain fully positively charged across pH ranges, Nuvia wPrime 2A Media’s weak AEX component can be modulated by adjusting the buffer pH, enabling milder elution conditions and increased control over binding/elution behavior. The Nuvia polyacrylamide bead matrix is engineered for chemical and mechanical resilience, maintaining dynamic binding capacity even under demanding flow rates, harsh cleaning (including NaOH), or exposure to strong solvents.
PhysioMimix Core (CN Bio)

Organ-on-a-chip system combining single-organ, multi-organ and higher-throughput configurations within one scalable platform
The drug discovery and development sector has reached an inflection point, with recent moves by the FDA paving the way for regulators worldwide to embrace the potential of new approach methodologies like organ-on-chip. Built on the industry-leading PhysioMimix brand, leveraging over a decade of organ-on-chip expertise, PhysioMimix Core combines the capabilities of CN Bio’s suite of instruments within an all-in-one system. Offering easy transitions between three performance-validated configurations for single-organ, multi-organ and high-throughput of up to 288 samples per run, the core system allows researchers to easily adopt, adapt and scale their workflows as experiments evolve. The benchtop design has a range of features, including tubeless microfluidic engineering to reduce contamination risk and maintenance requirements, recirculating media and adjustable inter/intra-organ flow rates for long-term studies, and ample material recovery for multi-omics analysis.