Scientists from the Pritzker School of Medicine, University of Chicago, have developed a thermoresponsive polymersome that can carry proteins and siRNA to the right cells without the need for complicated manufacturing steps or toxic chemicals.
Polymersomes are more stable than lipid nanoparticles and more flexible in the types of molecules they can carry, but typically require complicated processing steps, such as filtering, concentrating, and removing solvents, which are expensive and can damage delicate drugs. The Pritzker team’s solution was to design block copolymers – special polymers that can self-assemble into polymersomes when warmed from refrigeration temperatures to room temperature. The change in temperature causes the materials to organize themselves into uniform vesicles of around 100 nanometers in size.
The Pritzker method simplifies the process by mixing the polymers with the drug payload in cold water and warming the solution so that the particles form naturally with no need for purification. The materials can then be dried into a powder for easy storage and later reconstituted without losing effectiveness.
“Owing to high loading efficiency and homogeneous particle sizes upon warming,” the study says, “we can bypass tedious processing such as size exclusion and membrane filtration. Furthermore, the high solids content remains a highly attractive feature of our system, as current polymer and LNP technologies rely on further processing for concentration of suspensions to achieve a robust biological response.”
The polymers were specially designed with charged components that attract and hold onto negatively charged payloads, such as proteins and siRNA. As a result, the team achieved loading efficiencies of 75-99 percent, whereas many existing systems often fall below 20 percent. This efficiency reduces the amount of polymer needed for treatment and eliminates the material loss that typically comes with filtration steps. Plus, it simplifies dosing and helps maintain consistent quality from batch to batch.
The polymersomes were tested in multiple living systems to prove they work. In vaccination, they were used to deliver a model protein (ovalbumin) in mice, with and without the addition of a known immune-boosting agent. The encapsulated protein generated strong immune responses, both in terms of antibody production and T-cell activation. One dose triggered antibody levels that remained high a year later.
In an allergy tolerance test, mice receiving polymersomes carrying the allergen protein showed reduced signs of allergy, including lower inflammation and reduced levels of IgE (an allergy-related antibody). This suggests the polymersomes could be used not just to boost immunity, but to induce immune tolerance, a strategy useful for autoimmune diseases and allergies.
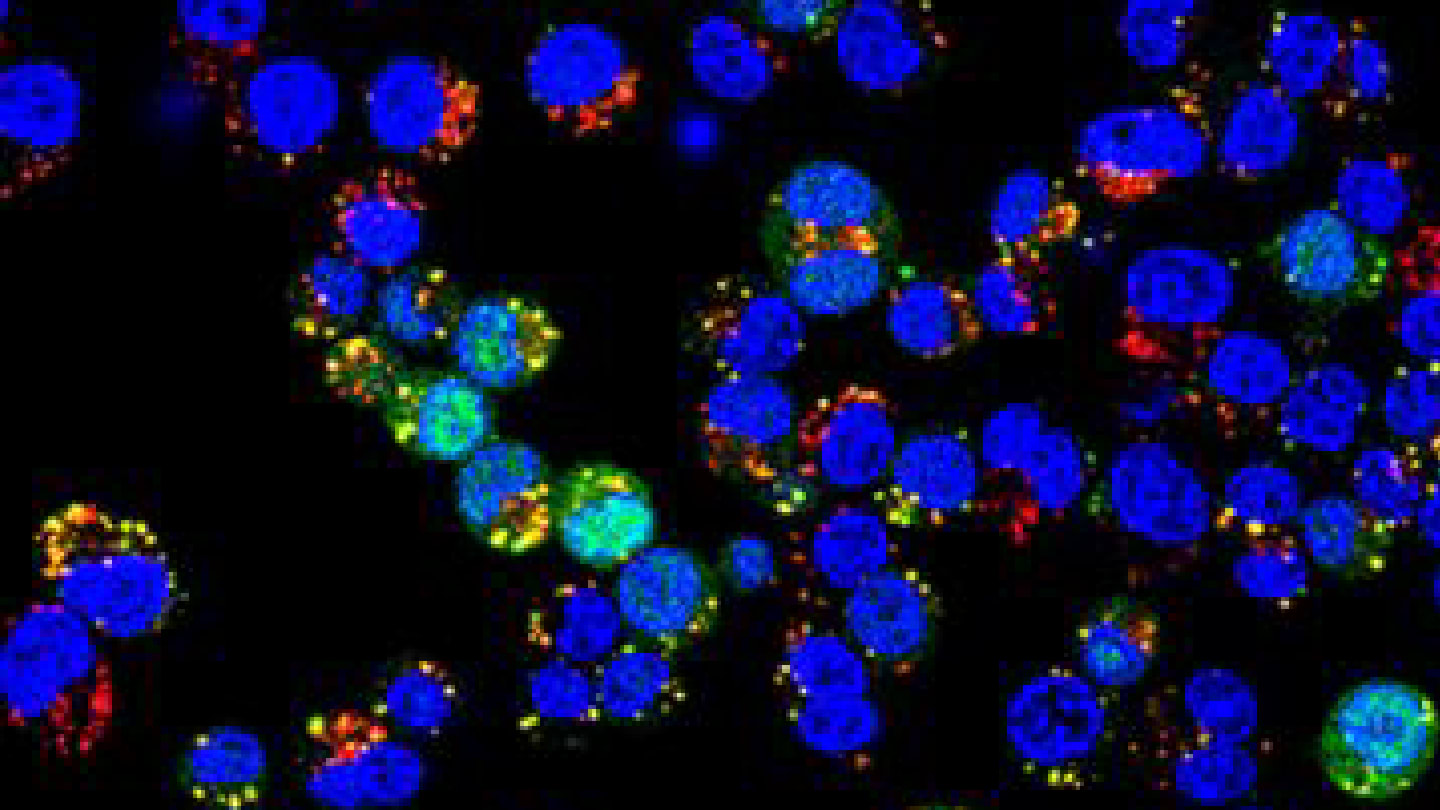
A third test involved using the polymersomes to deliver siRNA that silences cancer-promoting genes in breast cancer cells. Again in mouse models, the polymersomes successfully shut down target genes and slowed tumor growth. When compared head-to-head with commercial LNP formulations, the polymersomes performed better, particularly in intratumoral (direct tumor) injections.
Barry L. MacLean Professor for Molecular Engineering Innovation and Enterprise at UChicago Pritzker Molecular Engineering and a staff scientist at Argonne National Laboratory, said, “What excites me about this platform is its simplicity and versatility. By simply warming a sample from fridge temperature to room temperature, we can reliably make nanoparticles that are ready to deliver a wide variety of biological drugs.”




