
Injectable drugs are on the rise – driven by blockbuster biologics, next-generation therapies, and surging demand for patient-centered care at home. But while the science of self-administration is evolving rapidly, bringing these innovations to market and into patients’ hands remains a complex endeavor.
In a recent global survey commissioned by West Pharmaceutical Services, healthcare providers and patients alike expressed strong interest in self-injectable medicines and wearable delivery systems. Yet as new classes of therapies like GLP-1s gain momentum, the pressure is mounting to not only develop smarter, more flexible devices, but to ensure they’re scalable, user-friendly, and accessible across real-world settings.
In this follow-up discussion, West’s Anya Harry and Sanjeev Seenath reflect on what’s fueling the growth in injectable drug delivery, what’s getting in the way, and what types of technologies the future will demand.
Meet the Experts

Anya Harry – Chief Medical Officer and Vice President of R&D in Applied Research and Clinical Affairs

Sanjeev Seenath, PharmD, Manager, R&D & Clinical Development
Why are injectable drugs such a fast-growing drug segment? What trends are at play?
Pharmaceutical innovations have significantly advanced the treatment of many diseases, including cancer and chronic inflammatory diseases. By addressing the root biological underpinnings of disease, therapies have greatly improved the prognosis for patients, bringing symptoms well under control, and in some cases, extending lives. This fosters a strong need among both patients and HCPs for alternative, efficient ways of receiving these medicines.
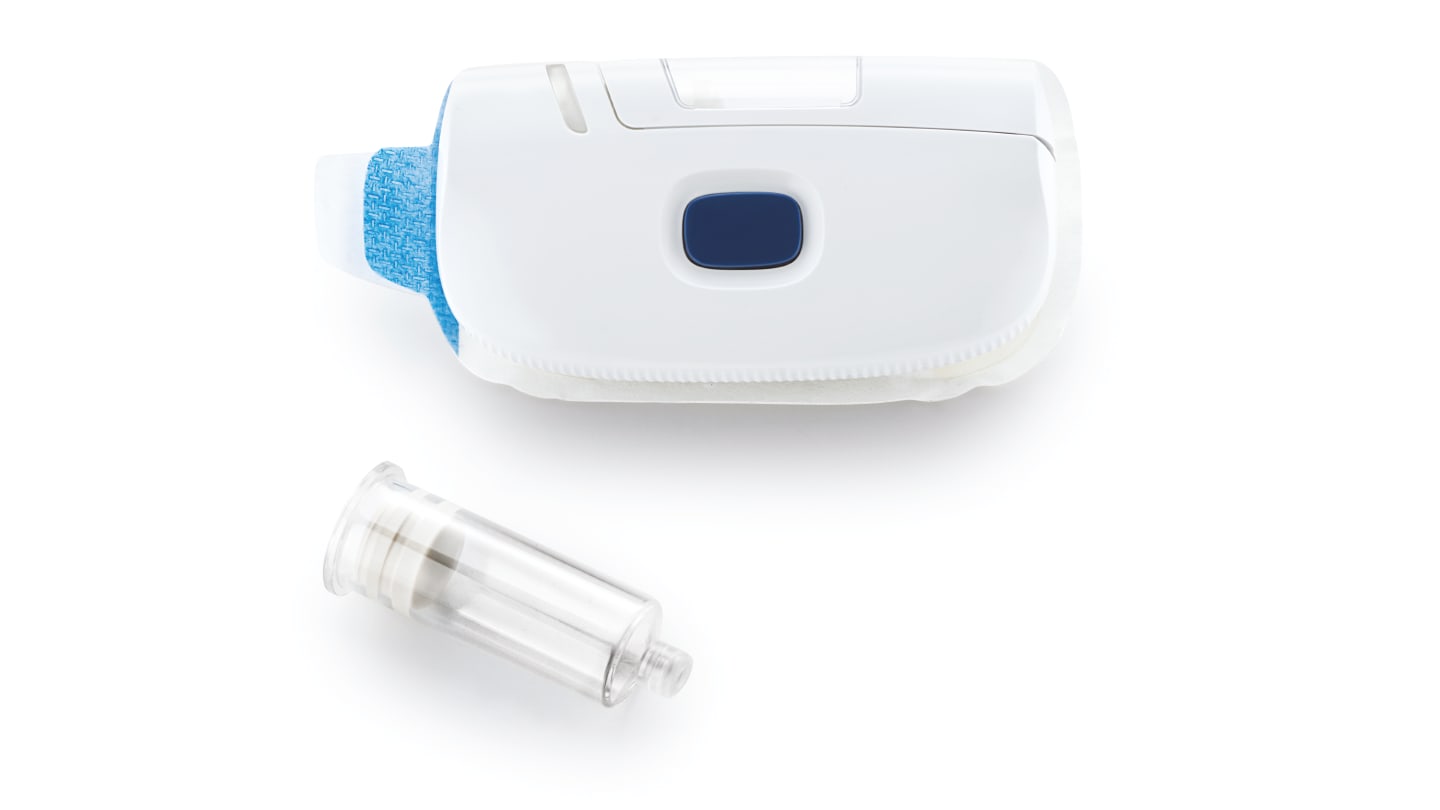
Right now, the industry is experiencing a shift from hospital to home for the management of chronic diseases – and increasing patient demand for more convenient treatment options. With the expansion of home-based healthcare, patients are interested in using advanced technologies, such as self-injectable drugs and OnBody Delivery Systems (OBDS) – and excited about the convenience and autonomy they could gain by administering medications themselves, rather than having to travel to a doctor’s office or infusion center.
Patient-centric healthcare models emphasize autonomy and ease-of-use. At the same time, advances in smart injectors that integrate Internet of Things (IoT) connectivity for precise and adjustable dosing and adherence monitoring can seamlessly link to digital health ecosystems. Potentially, this can also offer solutions for remote regions. Furthermore, the focus on sustainability is leading to the development of environmentally friendly materials for delivery devices.
How is the demand and hype around GLP-1s affecting the market and the drive for innovation in injectable drug delivery?
As the healthcare industry is evolving and growing in its need for more patient-focused drug delivery solutions, access to development and commercialization services to support popular products like glucagon-like peptide (GLP-1) becomes increasingly critical.
Whilst originally developed for diabetes, GLP-1 receptor agonists induce significant weight loss, and with obesity affecting more than 1 billion people globally, GLP-1s represent the first meaningful drug treatment for this chronic condition in many decades.
What is equally exciting are the readouts from clinical trials demonstrating a reduction in symptoms of heart failure and the risk of heart attacks and strokes – the most compelling evidence yet that the drugs have major benefits beyond weight loss itself. GLP-1s are also in clinical trials for chronic kidney disease, liver disease, Alzheimer’s disease, sleep apnea and osteoarthritis, broadening their application landscape significantly.
With the increased demand, these medications put even more pressure on our industry to ensure continuity of supply and quality.
There is plenty of innovation in drug delivery devices at technology companies and in academia. How challenging is it to get innovative devices commercialized and used in the real-world?
The commercialization and real-world adoption of innovative drug delivery devices presents several challenges. There is demand from patients and healthcare providers for new devices, but the regulatory environment is becoming more complex. Agencies such as the FDA and EMA must ensure device safety, efficacy and quality. For devices intended for home use or those with connectivity features for tracking and trending health data, and/or facilitating the exchange of healthcare information and interoperability, manufacturers must address ensure data can be shared consistently between different systems, and address concerns such as usability in non-clinical settings and cybersecurity vulnerabilities. Data management strategies must comply with privacy regulations like HIPAA to protect patient information, and the data must also be able to be shared.
Innovative devices also usually employ cutting-edge technologies, which requires significant R&D investment. Integrating new technologies, such as smart sensors or IoT capabilities, for example, presents compatibility and reliability challenges. Ensuring the device is user-friendly, particularly for patients who are not tech-savvy, is also crucial to success in the market.
Moreover, producing innovative devices at scale is a challenge. Manufacturers need to establish efficient production processes that maintain quality, while keeping costs manageable. Supply chain logistics, including the sourcing of advanced materials and components, also play a critical role in successful commercialization.
Even with advanced features, gaining market acceptance can be challenging. Devices must not only meet clinical needs but also align with patient preferences and healthcare provider practices. Effective marketing strategies, education, and training are all essential to drive adoption.

What type of new drug delivery options are needed?
As the healthcare landscape continues to evolve, there is an increasing demand for novel drug delivery options that prioritize ease-of-use, comfort, and flexibility. Both healthcare providers and patients are seeking advanced solutions, particularly in the areas of oncology and immunology, where the ability to administer highly viscous formulations and large volume doses is essential. These sectors underscore the need for drug delivery systems that enable self-administration, empowering patients with chronic conditions and compromised immune systems to manage their treatments more autonomously.
Industry stakeholders are seeking key features and advancements, such as enhanced device capabilities that are compatible with a wide range of high-viscosity and high-volume formulations. Additionally, robust platforms with a broad design window to accommodate different delivery speeds are essential.





