
Acting on the Drug Supply Chain Security Act
The US drug supply chain is not immune to counterfeits – so here’s what we can do about it
Shabbir Imber Safdar | | 8 min read | Discussion
The author of this article is a member of the Partnership for Safe Medicines (PSM).
The PSM, founded in 2003, is a not-for-profit focused entirely on researching the dangers of counterfeit drugs in America and educating the public about how to stay safe. PSM is an active member of the Fight the Fakes Alliance (1), a multi-stakeholder nonprofit association that aims to raise awareness of the dangers of falsified and substandard medicines.

Counterfeit medicine is an issue faced by every country on the planet. In 2018, the World Health Organization estimated that one in 10 medical products in low- and middle-income countries is substandard or falsified (2). In the US, a series of supply chain breaks in the opening years of the 21st century convinced lawmakers that the country’s drug supply chain was in need of improvement. In 2020, the European Union Intellectual Property Office and the Organisation for Economic Cooperation and Development estimated that the total value of counterfeit medicines sold throughout the world is over US$4 billion annually (3).

This bottle received a serial number on the manufacturing floor, which every other handler recorded on its way to the pharmacy shelf.
Fake labels and shady wholesalers
In 2002, a 16-year-old transplant patient in New York was prescribed 40,000 unit/mL Epogen to combat post-surgery anemia (4). The vials of medicine a local pharmacy provided were labeled as such – but those labels were forged. A few months earlier, 110,000 low-dose vials of Epogen were sold to a small pharmacy in Miami, Florida, over 2000 kilometers away from where the transplant patient lived. The pharmacist had no intention of dispensing them to patients and instead sold them on. The buyer was a counterfeiter.
The counterfeiter took the temperature-sensitive vials, left them to soak overnight, rubbed off the original manufacturer’s labels, and applied new fake labels indicating that the vials contained 40,000 unit/mL Epogen. Now, those low-dose vials that had originally sold for $25 were worth $470 each, making the scheme worth $46 million. The vials made their way through increasingly legitimate wholesalers until they reached the original wholesaler in Arizona (over 3700 kilometers from Miami), who thought they were real and placed them with the rest of its high-dose Epogen stock. A national pharmacy chain then purchased the vials, which is how a 16-year-old transplant recipient in New York received them. Each injection caused the teenager such extreme cramping and spasms that the young man would scream so loudly that it terrified his younger sister (5).
Loose licensure regulations and the simple act of replacing the labels on the vials allowed this counterfeiting scheme to occur. To prevent something like this from happening again, the US had to improve the security of its drug supply chain.
Enter the DSCSA
Enacted by Congress on November 27, 2013, the Drug Supply Chain Security Act (DSCSA) outlined the critical steps needed to establish interoperable electronic tracing of prescription drug products at the package level by 2023. It also established national standards of licensure for wholesale drug distributors and third-party logistics providers (3PLs) (6). Under the DSCSA, all prescription drug transactions must include the following three items:
- Transaction Information (TI): identifying information, including the name of the drug, strength, dosage, NDC code, container size, number of containers, and lot numbers.
- Transaction History (TH): a historical record of all of the entities that have been in possession of any particular prescription drug back to and including the original manufacturer.
- Transaction Statement (TS): certifies that the entity transferring ownership is authorized by the DSCSA to do so, received the product from a person authorized by the DSCSA to do so, did not knowingly ship a suspect or illegitimate product, and did not knowingly provide any false transaction information or transaction history.
The DSCSA specifically states that shipments for prescription drugs without TI, TH, and TS should be refused and due diligence requires that all participants in the drug supply chain must confirm that they have not received any illegitimate product.
The Gilead Sciences HIV Medicines Case
In August 2021, Gilead Sciences released a statement warning the public about counterfeit versions of two of its HIV medications – Biktarvy and Descovy – circulating within the US drug supply chain (7). The counterfeiters were refilling genuine Gilead bottles with incorrect tablets and selling the bottles to pharmacies. Though this statement may have given the public the impression of an incipient threat, a court document made public months later showed that Gilead Sciences had already filed a civil suit against over 75 companies and individuals it claimed participated in a massive counterfeit prescription drug trafficking ring (8).
Gilead Sciences became aware of this counterfeiting operation through a series of complaints from both pharmacies and patients in the months before filing the civil suit. Additionally, AmerisourceBergen, one of only 16 Gilead-authorized distributors for Biktarvy and Descovy (9), reached out to Gilead Sciences with transaction histories that falsely listed AmerisourceBergen as having purchased various bottles.
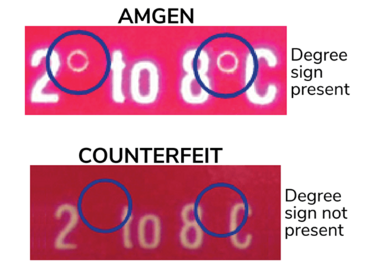
As part of the DSCSA, every bottle of medication sold in the US must have its own unique serial number. In this case, the counterfeiters allegedly used those numbers to add a layer of believability to their fakes. Pharmacists are known for paying attention to details, and perfectly counterfeiting a bottle is hard to do. The same cannot always be said for a paper pedigree. In this case, the counterfeiters simply lied on the required transaction history form. Unless a pharmacist checked the transaction log for a break in the chain, then the fake form would not draw as much attention as a fake bottle.
Gilead Sciences went so far as to hire an outside laboratory to examine some of the seized bottles. The laboratory confirmed that legitimate Gilead product bottles were being reused and that replica seals and adhesive that did not match the original foil and adhesive were used to make the authentic bottles look like they had never been opened. This testing confirmed what the DSCSA data were saying: these once-real bottles were now counterfeit, filled with incorrect pills, and the transaction histories had been falsified.
In relation to this case, a small-town community pharmacist in Texas received an offer to buy HIV medicine from one of the wholesalers named in the Gilead lawsuit. The price was really good. In fact, it was better than the price he and his fellow pharmacists were getting with their buying group, so he placed an order. In the US, pharmacies often band together in a large purchasing contract, using volume purchases to get the best prices. AmerisourceBergen had told the pharmacist his buying group was big enough to get the very best price.
However, when the medication arrived, he looked at its transaction history. According to the document, it had passed through AmerisourceBergen, which was his wholesaler! Even worse, this secondary wholesaler who bought from AmerisourceBergen, must be getting a better price than his buying group because it was cheaper.
The pharmacist sent a copy of the transaction history, the serial numbers of the bottles, and the invoice to AmerisourceBergen, asking why he was not, in fact, getting the very best price. AmerisourceBergen checked their electronic records and discovered that the bottles in question had never been in their warehouse. That meant the sales log was fake – and that the medicine was likely fake too.
Upon confirmation from AmerisourceBergen that the product was counterfeit, the pharmacist in Texas quarantined the medicine to prevent any patient from receiving it and received a refund from his supplier. Although no information has been shared to date on how many of the counterfeited bottles were distributed to pharmacies or reached patients, it is known that over 85,000 bottles were seized by investigators over the course of Gilead Sciences’ investigation (10).
DSCSA: already protecting patients
The implementation of the DSCSA and its reliance on electronic records meant that it was possible for pharmacists and wholesalers to check the legitimacy of a product quickly when needed. At the Partnership for Safe Medicines, we believe this may be the first known incident in which patients were protected using the DSCSA. As the Gilead case developed, the manufacturer was able to compare transaction history data with the serial numbers and quickly see that the entire transaction history was fabricated. Additionally, because they had access to real bottles, they could work out who they thought the members of the prescription drug trafficking ring were. Before DSCSA data, a faked pedigree was enough for counterfeits to be slipped into the system; now, it is much more difficult for counterfeit medicines to hide when all the information is lined up.
A study of prosecutions of illegally online pharmacies, who often traffic in counterfeit medicines, demonstrates that there are significant profits and limited penalties (11).
This dynamic means that criminals will always be tempted to counterfeit prescription drugs and, recently, their activity in the US has been very evident. Here are some recent news stories:
- The Pharmaceutical Security Institute warned that some Mexican pharmacies were selling counterfeit blood thinners packaged in English (12) to draw the business of American tourists visiting Mexico. This comes in parallel with a steady flow of counterfeit alerts from Mexico’s drug regulator, COFEPRIS (13).
- Johnson & Johnson filed suit against a group of distributors – many the same as those named in the Gilead case – for counterfeiting several of its HIV treatments: Symtuza, Prezcobix, Prezista, and Edurant (14).
- Gilead brought an action against a drug diversion ring in Florida that allegedly recruited patients to participate in the company’s medical assistance program to acquire low-priced Truvada and resell the diverted product for a profit on the black market (15).
Implementing the DSCSA has been a long process that required substantial upfront investments from companies across the US pharmaceutical supply chain. DSCSA data and serialization have already taken thousands of counterfeit bottles of medicines out of the US drug supply chain. And for that, we should all be grateful.
- Fight the Fakes Alliance, “Fight the Fakes” (2022). Available at: https://bit.ly/FtheF.
- WHO, “Substandard and falsified medical products” (2022). Available at: https://bit.ly/sub-med-who.
- EUIPO, “Trade in Counterfeit Pharmaceutical Products” (2022). Available at: https://bit.ly/eu-coun-ph.
- The Partnership for Safe Medicines, “August 27, 2020 Video: Behind The Scam: When Labels Lie” (2020). Available at: https://bit.ly/labels-lie.
- The Boston Globe, “Counterfeit Drugs Leave Real Casualties (2005). Available at: https://bit.ly/3x8lH6C.
- FDA, “Drug Supply Chain Security Act (DSCSA)” (2022). Available at: https://bit.ly/DSCSA-fda.
- Gilead Sciences, “Gilead Warns of Counterfeit HIV Medication Being Distributed in the United States” (2021). Available at: https://bit.ly/gil-hiv.
- United States District Court Eastern District of New York, “Case No. 21-cv-4106 (AMD) (RER)” (2019). Available at: https://bit.ly/ny-gil.
- Gilead Sciences, “Authorized Distributors” (2021). Available at: https://bit.ly/auth-dist.
- J Walker, “Drugmaker Gilead Alleges Counterfeiting Ring Sold Its HIV Drugs,” Wall Street Journal (2022). Available at: https://on.wsj.com/3wBl8C6.
- C Guerra et al., “USA criminal and civil prosecutions associated with illicit online pharmacies: legal analysis and global implications,” MA@PoC (2017). Available at: https://bit.ly/ill-ph-us
- The Partnership for Safe Medicines, “Fake Blood Thinners Found In Mexican Pharmacies” (2022). Available at: https://bit.ly/blo-mex.
- Government of Mexico, “Alertas sanitarias de medicamentos” (2022). Available at: https://bit.ly/mex-gov.
- B Pierson, “J&J sues to block sales of ’dangerous’ counterfeit HIV drugs” (2022). Available at: https://reut.rs/3wp9nzN.
- Z Becker, “Gilead presses HIV drug fraud in court, wins freeze of ’kingpin’ assets” (2022). Available at: https://bit.ly/gil-pin.
Shabbir Imber Safdar, Executive Director, Partnership for Safe Medicines and Fight the Fakes Alliance’s Executive Board member



















