
Chasing Checkpoints
Therapeutic modulation of checkpoint signals is bringing new treatments to cancer patients – and the industry is scrambling to find more.
Checkpoint inhibitors have been identified as one of the most exciting areas of progress in the industry (1) – and with good reason. A huge amount of research from academia and industry is focusing on the potential of checkpoint inhibitors for treating cancer – and a handful of therapies have already launched. What is a checkpoint inhibitor? Many cancers produce mutant proteins that are recognizable as “foreign” by the immune system. Often, however, tumor cells also have the ability to push molecular buttons – “checkpoints” – that inhibit anti-tumor immunity. But when one door closes another opens; the elucidation of checkpoint-mediated inhibition has led to a new class of therapies called checkpoint inhibitors designed to block tumor-mediated inhibitory signals, thus allowing the immune system to mount a more effective anti-tumor response.
The potential of immunotherapy in cancer treatment has been discussed for well over one hundred years. In the 1880s, William Coley published a paper describing how he’d injected cancer patients with streptococcal cultures and observed tumour regression in some cases – but he didn’t know why. What we have today that Coley didn’t is a better understanding of immunotherapy, how T cells work and how cancer evades the body’s natural immune system – although there is still a long way to go (2).
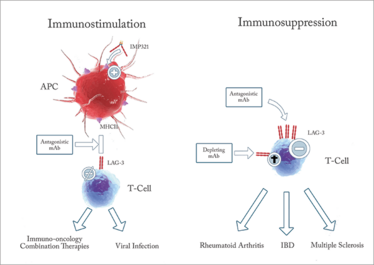
Figure 1. Targeting LAG-3 may lead to multiple therapeutics in numerous indications including immune-oncology, rheumatoid arthritis, inflammatory bowel disease and multiple sclerosis. IMP321 is under investigation by Prima BioMed.
Inhibiting inhibition
I’ve spent most of my career focusing -interesting to see how the times have changed. Back when I entered the field, immunology was little more than a sub-specialty of infectious disease. During my fellowship in the 1970s, I was very focused on human tumor-infiltrating lymphocytes (TILs – implicated in killing tumor cells), which was quite unique at the time given that the vast majority of immunologists were working with mouse cells. I cloned human T cell receptors and elucidated how they could recognize two different determinants, the antigenic peptide and the major histocompatibility complex. In the 1990s, my group at Institut Gustave Roussy (France) discovered that 10-30 percent of TILs from different metastatic human tumors were of identical specificity, showing that they had recognized a tumor-specific peptide and multiplied locally (clonotypic expansion). This in turn suggested that disinhibiting this population could generate an anti-tumor response, and led to my work on checkpoint inhibitors.
Today, immunology is a vibrant area of research, and cancer immunotherapy, in particular, continues to go from strength to strength. At first, much attention focused on blocking the CTLA-4 checkpoint, which had benefits in certain groups of patients, but responses were not universal and there could be significant toxicity in some cases. So far, there has only been one FDA approval of a CTLA-4 checkpoint inhibitor: ipilimumab is a monoclonal antibody that was first approved in 2011 for treating skin melanoma – and was the first new treatment for metastatic melanoma in more than a decade. The treatment, however, can have serious side effects (3), which has spurred drug developers to look for alternative options.
Today, a lot of checkpoint inhibitor activity has focused on programmed death ligand 1 (PD-L1) and its receptor, programmed death 1 (PD-1). PD-1 is located on T cells while PD-L1 is found on normal cells to prevent them from being attacked by T cells. PD-L1 is also found on some cancer cells. Drugs targeting PD-1/PDL-1 have been approved by the FDA in recent years – atezolizumab, nivolumab and pembrolizumab – have good response rates, but side effects can still be serious for some patients. Many more PD-1/PDL-1 drugs are being developed, but the industry is also looking to develop drugs that interfere with other checkpoints, such as lymphocyte activation gene-3 (LAG-3), killer immunoglobulin like receptor (KIR), and T-cell immunoglobulin and mucin domain-3 (TIM-3) (4). Of these, LAG-3 is receiving growing interest. A number of pharma companies are investigating LAG-3; Novartis, Merck, Regeneron, Boerhinger-Ingelheim and Bristol-Myers Squibb all have LAG-3 products in the clinic. BMS’s anti-LAG-3 has been in the clinic for more than three years, and the company started five new large clinical trials last year. In fact, more than 2,000 patients are being treated with anti-LAG3.
Business LAG
Academic research is pretty competitive and you have to fight for your ideas. From 1990 until 2002, my group was the only one publishing on LAG-3. At times like this, when you really believe in the potential of something, it’s important to be resilient. I knew that if the immune system could recognize tumors, then it would be an important advance – potentially we could wipe out even large tumor masses. And the results I was seeing with LAG-3 were compelling.
During the 1990s, I was director of a unit at INSERM – the Institut national de la santé et de la recherche médicale in Paris, France. INSERM gave the LAG-3 patents to Serono, but Sereno were not focused on moving it forward at the time. I wanted to get things moving so I quit my immuno-oncology professorship at the university with the aim of getting the patents back and starting up a new company – Immutep. Along the way, I learned just how inefficient the biotech world is. More than three years of negotiations were required to licence the patent back from Serono, and obtaining Series A funding from investors required immense effort. Series A was the last tranche raised; funding rounds B and C were never forthcoming, and a cash-strapped Immutep was forced to license some antibody products to GlaxoSmithKline and Novartis – after all, without money, you can’t accomplish anything.
But it turned out well in the end. Immutep was acquired by Prima BioMed in 2014 – and today I’m the Chief Scientific and Medical Officer. At the time, Marc Voigt, CEO of Prima BioMed, wasn’t looking for LAG-3, specifically – he was simply interested in promising innovation focused on immuno-oncology, but the GSK and Novartis deals, as well as the advanced clinical development and potential of another product, caught his eye and validated the work that we were doing at Immutep. Today, my work with LAG-3 continues at Prima BioMed and the two licensed products are progressing well in clinical trials. We also have a third product in development – IMP321 for metastatic breast cancer and metastatic melanoma. Notably, we are the first company to have a Chinese-made biological enter clinical trials in Europe and the first one presenting – just very recently – an agonist antibody to LAG-3.
Such progress is very rewarding to see, given that I was the one who discovered LAG-3 in the 1990s (5). I’ve dedicated a large part of my career to not only understanding LAG-3, but trying to make it a reality. A number of researchers have also confirmed that LAG-3 could play a role in the cancer therapies of the future (6)(7). LAG-3 has a unique bi-faceted regulatory role – it participates in the negative regulation of T cells (such as cellular proliferation and activation), similarly to CTLA-4 and PD-1, but it also positively regulates antigen-presenting cells. So it could be possible to create a range of LAG-3-based products, not just in oncology but also in autoimmune disease. In oncology, LAG-3 can be used in combination with PD-1 to help with metastatic cancer – potentially providing a 50-percent response rate. At present, achieving this level of response in metastatic cancer requires combining anti-PD1 with anti-CTLA4, which is relatively toxic.
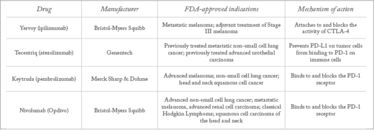
Table 1. Approved checkpoint inhibitors.
We’ve only just begun
We have only scraped the surface of the potential of checkpoint inhibitors for drug development. Many people in the industry still don’t really understand T cells or dendritic cells (and explaining the science to investors is always a challenge), but they do understand durable tumor regression and how important it is. The excitement and expectations for the field are having a very positive impact in terms of greater funding, even for more challenging approaches to immunotherapy, such as CAR-T cells. Indeed, the widely reported successes with CAR-T therapies have also helped to put immunology in the spotlight.
All of that said, I think immunology still deserves yet more attention. Many of the chronic diseases that the industry is desperately trying to develop treatments for can be approached from an immunological perspective. In many cases, disease is caused because of immune system regulation. Of course there are other factors that play a role too, such as genetics and external influences, but in general I believe that the full potential of immunology has yet to be uncovered and many new discoveries are waiting to be made.
In the future, immunotherapy will be increasingly broadly applied, not only in oncology and autoimmune disease, but also in neurodegenerative diseases, such as Parkinson’s and Alzheimer’s. There is also potential in less obvious conditions, such as atherosclerosis. Many chronic conditions would benefit from approaches that are more fundamental than merely treating symptoms. For example, could the use of statins for high blood pressure eventually be replaced by an immunology-based approach to the underlying problem?
Frédéric Triebel is Chief Scientific and Medical Officer of Prima BioMed, New South Wales, Australia.
- S Sutton, “Revelations and Resolutions”, The Medicine Maker, 26, 18–26 (2017). Available at: bit.ly/2l6p8ba.
- CR Parish, “Cancer immunotherapy: The past, the present and the future”, Immunology and Cell Biology, 81, 106–113 (2003). PMID: 12631233
- LA Fecher at al., “Ipilimumab and Its Toxicities: A Multidisciplinary Approach”, Oncologist, 18, 733-743 (2013). PMID: 23774827
- H El-Osta, “Immune checkpoint inhibitors: the new frontier in non-small-cell lung cancer treatment”, Onco. Targets Ther., 9, 5101-5116. PMID: 27574451
- F Triebel, “LAG-3, a novel lymphocyte activation gene closely related to CD4”, J Exp. Med., 1;171, 1393-1405 (1990). PMID: 1692078
- X Li et al., “Emerging immune checkpoints for cancer therapy”, Acta Oncol., 54, 1706-1713 (2015). PMID: 26361073
- ES Kim et al., “Immune Checkpoint Modulators: An Emerging Antiglioma Armamentarium”, J. Immunol. Res., Epub Jan 4 (2016). PMID: 26881264
Frédéric Triebel is Chief Scientific and Medical Officer of Prima Biomed, New South Wales, Australia.



















