Digitalization Now
Digital technologies are here – but are they being used adequately?

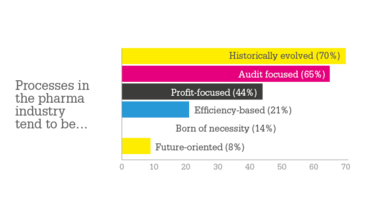
The pharma industry could not operate without regulations – drug development is complex and regulations ensure companies pay adequate attention to all essential aspects, ultimately ensuring patient safety. But many also perceive regulations as stifling innovation. In its study, “Pharma Insights 2019”, consulting firm MAIN5 found that 65 percent of processes in a pharma company are audit-focused; only 8 percent of processes are future oriented. Digitalization could have a huge impact on processes – if companies can overcome the challenges of implementation. We spoke with Tore Bergsteiner, Managing Director at MAIN5, to learn more about the survey respondents’ attitudes to new technologies.
What were the most surprising findings of the study?
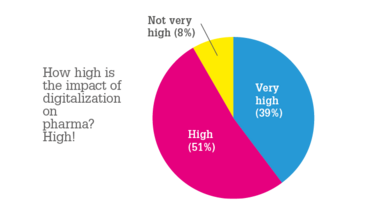
We were astonished by how massive the impact of digitalization could be in life sciences (90 percent of respondents said the impact of digitalization on the pharma and life science sectors is high or very high). However, processes in pharma are still perceived as being primarily audit-focused, or even just historically evolved. Even though the digital transformation is present, it is currently not matched with a substantial change in the processes and cultures within pharmaceutical organizations. In particular, pharma is a rather data driven business and we can see high potential in the value that could come from computational mass analysis of the large data repositories.
We assume that the situation will change in the next 2-4 years.
Given the highly regulated nature of the pharma industry, it is perhaps unsurprising that most companies operate from audit to audit. How do you think pharma companies can shift away from this mindset while still adhering to regulations?
Some organizations have already started changing. Rather than a culture of internal, written down over-regulation, some are moving towards the concept of establishing a quality culture by giving more responsibility to each individual employee. This movement is accompanied by establishing a guiding principle called lifelong learning. In pharma, we see that QA and HR are collaborating to establish global learning management systems.
Pharma has so much data at its disposal – why is it so challenging for companies to use their data?
There are probably three main reasons why it is so challenging to utilize data to achieve a real business advantage:
- The data formats are not consistent across the repositories and can hardly be matched to automatically generate meaningful statistics or fully automated results.
- The quality of the data has not yet been checked against the best way of using them. The data cannot be used for the intended goals, if the data accuracy is not assured.
- Due to the complexity of the industry, we don’t yet have the knowledge of how best to use data material. Companies realize that it might be a treasure – and it surely is – but most need a manual to really see the possibilities.


Other industries have been talking about the value of data and digital technologies for many years. How far does pharma lag behind?
To be honest, I believe that most industries are struggling with the challenge that digital transformation brings. Some industries, such as insurance, energy, automotive, defense and retail, are discreet regarding the range of problems in their businesses. In general, however, industries such as banking and pharma have to cope with an enormous amount of regulation all over the world, while others are free to “just do it.” It is hard to tell in terms of years how far the pharma industry may be behind, or how quick it may catch up. Certainly, the investments put into digitalization today will decide the market presence of organizations tomorrow.
For a company that is quite far behind – and operating from audit to audit – what are your top tips on how they can start to break the cycle?
The goal should be to create efficient and easy to understand processes without harming compliance. Sometimes it takes an outside eye to observe and realize the hiccups. Our consultants often find that companies are working historically – using processes that they have learned over the decades, even though there are rapidly changing tool sets available that can help (and are being overlooked).
What other findings from the survey stood out?
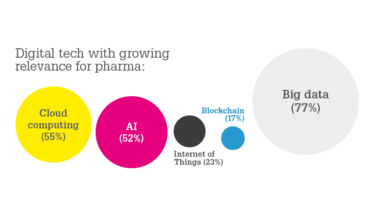
Cloud computing – the technology was envisioned as a threat 5-10 years ago. However, 55 percent of survey respondents perceived cloud computing as a true enabler today. This is an exciting new result! Cloud computing is also an enabler of artificial intelligence and big data because the cloud is needed to work in the immense and growing amount of data.
What are your thoughts on the future of digitization in pharma?
We often observe that new digital technologies – such as AI methodologies – are exciting because of what they seem to be. Instead of looking for a business case to use new digital technologies, companies should be looking at specific problems or business scenarios, and envisioning how sub-optimal processes could be improved in terms of quality, resources and quantity – in some cases, a carefully selected technology, such as AI, may be the solution but it depends on the problem. First, understand the problem; second, choose the most optimal automation tool that can help! There are a lot of compelling technologies out there. Our mission is to help manufacturers to better understand the options of the technology and to develop a strategy and roadmap for their businesses.

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















