
Embracing the Digital Transformation
How does digital transformation create a competitive advantage for customers and emphasize Bachem’s technical leadership role in the TIDES CDMO field?
Daniel Samson | | 3 min read
sponsored by Bachem

The fourth industrial revolution has started slowly in the pharma and biotech sectors, but more and more companies now embrace digital transformation in operations and supply chains (1). At the beginning, the drivers for digital innovation were cost savings and productivity, but there are many business cases beyond financial KPIs. For example, improved flexibility in capacity allocation and production scheduling can shorten time to market for new modalities, such as oligonucleotides. Oligonucleotides are a growing sector of the market, with 15 products already approved and more than 900 products in preclinical and clinical trials. Both the market and demand continue to grow, and digital transformation represents a great opportunity for CDMOs to keep pace with the dynamic landscape.
As the technology-leading CDMO for peptides and oligonucleotides, Bachem sees digitalization as an enabler to meet changeable demands, compliance, and ambitious timelines for high-quality APIs. Taking the automation pyramid (see figure 1) as a framework, digitalization projects are required in all layers in the digital transformation journey of pharmaceutical operations towards Industry 4.0.
One of our recent digital transformation lighthouse projects was the full automation of the industrial solid phase peptide synthesis (SPPS) process. To this end, the first Bachem robot-operator was designed and programed to execute the complete amino-acid activation and addition step. A key component of the automated equipment train is process analytical technology (PAT), which enables inline monitoring after key steps. With PAT, the need for manual in-process controls is omitted while ensuring real-time control of critical process parameters.
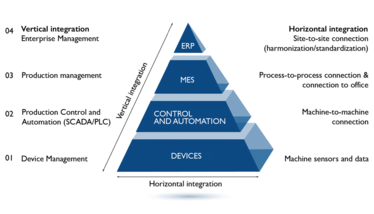
Figure 1. The automation pyramid for state-of-the-art manufacturing of peptides and oligonucleotides (TIDES)
In addition to full automation, we digitalized our SPPS process by integrating the control system of the production floor (levels 1 and 2; see figure 1) with a manufacturing execution system (MES, level 3), and the enterprise resource planning (ERP) system (level 4).
The MES has three major tasks:
- lead the process control system by defining the sequence of operations that have to be performed: Master Batch Record
- record all events, process values, and alarms as they happen during the process, and generate the electronic batch record
- manage equipment status (point- of-use and status verification, calibration, cleaning recipes etc) with integrated digital logbooks
In the context of vertical integration, the MES interconnects with our ERP system. Material verification, execution of process orders, creation of manufacturing orders, automatic stock creation, and material flow are all tasks that are now fully automated and paper-free. The risk of human error, such as material loss or mix-up, is omitted.
The process means that we can ensure that the correct material is used at the correct process step, as well as a reliable stock and inventory management generating a single source of truth in the ERP system.
All process data is logged in real time in the plant information (PI) system, which is a data Historian platform. This platform enables long-term archiving of process data, batch data, and facilitates data analytics, process optimizations, or root cause investigations. Both MES and PI systems are being rolled- out company wide.
Entering the era of pharma 4.0 demonstrates our commitment to our customers. Bachem has made huge steps in ensuring swifter interaction, more flexible production, and documentation sharing. With these newly integrated systems, we increase our capacity and speed by streamlining GMP documentation while boosting our processes’ efficiency regarding quality, cost, and time. Digital transformation will allow us to meet the increasing demand for new modalities and increase the speed with which these new therapies can reach the market. Thus, we will help our customers in their mission to reach large patient populations and to transform as many lives as possible.
CDMO: Contract Development and Manufacturing Organization
- G Hole et al., International Journal of Pharmaceutics: X, 3 (2021). DOI: 10.1016/j.ijpx.2021.100095
Daniel brings 15 years of industry experience to the team, with an emphasis on TIDES process R&D, manufacturing and CMC development. He leads Bachem’s oligonucleotide unit including innovation projects, R&D and manufacturing activities. Daniel holds a PhD in organic chemistry from the University of Konstanz, and an MBA from the International Institute for Management Development (IMD), Lausanne. In previous stages of his career he was a lab head for process optimization, technology transfer, Quality by Design, and scale-up of synthetic peptide manufacturing procedures. From 2012, Daniel was a Vice President API Manufacturing and had full responsibility for all large-scale solid phase peptide and oligonucleotide syntheses, downstream operations, and CMC activities within Bachem AG. On March 1, 2022, Daniel assumed a new position as Vice President, Head
Oligonucleotides at Bachem.



















