Five Steps You Won’t Want to Miss When Filing an IND
Set yourself up for success by asking these questions along the IND application process.
sponsored by Regis
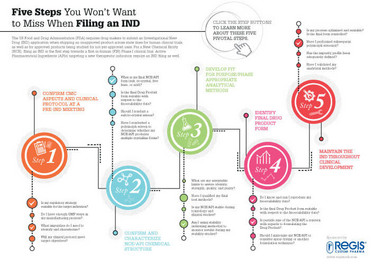
The FDA requires drug makers to submit an Investigational New Drug (IND) application when shipping an unapproved product across state lines for human clinical trials, as well as for approved products being studied for not-yet-approved uses. For a New Chemical Entity, filing an IND is the first step towards a first-in-human Phase I clinical trial. Active Pharmaceutical Ingredients targeting a new therapeutic indication require an IND filing as well.
What are the most important things to consider when filing an IND? This infographic, sponsored by Regis, sets out the five key steps to success.