Global Recall
It is the nightmare scenario for any drug firm. And yet, in a world of regulatory rigor and complex supply chains, product recalls are becoming increasingly challenging. Here’s how you can protect your company and customers, should the worst happen.
The increasing globalization of the pharmaceutical supply chain has caused a headache for drug manufacturers. Increased international regulatory collaboration and oversight, stricter requirements for imported products and raw materials, new sourcing and supplier verification processes, the need for traceability protocols... the supply chain is more complex than ever before. With all these different factors to keep an eye on, it’s perhaps no wonder that pharmaceutical product recalls have also significantly increased in complexity.

Globalization of recalls
No one is feeling the effects of the globalization rollercoaster more than Indian drug manufacturers. India has risen rapidly to become the number two exporter of pharmaceuticals to the US, but has hit a few ‘bumps’ along the way. A recent FDA Warning Letter to an Indian manufacturing facility was widely reported in the media after noting problems from falsification of data to “dead and decaying frogs” near the exit dock. Indeed, the FDA has been monitoring foreign drug companies much more closely and has banned multiple Indian drug makers from importing products to the US over the past year. Added to a series of FDA recalls for Indian pharmaceutical manufacturers, it’s clear that these are not just warning shots, but the result of strict ongoing oversight that is not likely to stop in the foreseeable future.
For me, the main lesson from these recall woes is that manufacturers must be prepared to implement quality control measures on a global scale, if they want to effectively compete in the global economy. They must also be prepared to handle subsequent recalls in a way that complies with regulations; for example, it’s a good idea to devise country-specific recall plans and on-site assessments to identify risks in standard operating procedures. Companies also need surge capacity to handle the complexity of large-scale, geographically distributed events. Companies are generally good at doing forward logistics and shipping, but may not be as evolved on the reverse side. Today’s recalls often affect more than one country, which makes the process more challenging. For instance, translation services or additional linguistic capabilities at call centers may be required. Product collection, storage, shipment and destruction will also vary between geographic regions based on local regulatory requirements and border control.
The sheer size of a major recall is often daunting enough, but when a manufacturer starts to look at the laundry list of other potential issues in the recall process (communication, logistics, supply chain management, and so on), it can seem like an insurmountable task. However, this ‘recall sprawl’ can be conquered by preparing for the worst in advance with a strong recall plan.
Recall planning: the importance of being earnest
There are 5-10 product recalls issued every day in the USA alone. And yet, I can’t tell you how many times I have given my card to the regulatory manager or CEO of a company and been told “We have a good process in place. We’ve never had a recall and I don’t think we ever will.” Weeks, months, or sometimes years later, I’ll receive a call: “We need some help…”
No manufacturer wants to believe that its product may be the subject of a recall, but to deny the possibility completely is a dangerous business; especially given that the best way for an organization to cope well in such a situation is to ensure that it is adequately prepared. It’s true that there is now more awareness about recalls, and most companies do have a recall plan. Whether it is an effective, comprehensive plan or not is a different story. Fortunately, there are some specific precautionary steps that all companies can take:
1. Involve the right people
In essence, an effective recall plan empowers organizations to quickly and efficiently locate recalled product and remove it from the marketplace. Your first task is to make sure you have the right people involved, from the planning stage onwards. It’s not just the quality and regulation teams that will have a role to play in a recall – you will need operations, sales and marketing, logistics, communications and others to play their part. Having robust discussions between departments before a crisis occurs is critical. Leaving out a key department at the planning stage can really hamper your efforts – for example, your sales teams can often be your most direct route to reach customers so they need to be kept up to speed. Another common omission is the finance department. The cost of recalls can be massive – by bringing finance into the discussion early on, you can identify ways to minimize costs without compromising patient safety.
2. Clearly define roles
To succeed, you must very clearly define the role and responsibility of each member of the recall management team. This section of the plan should also drill into the scope of authority and responsibilities of each individual, department, and affiliate, so that all members are clear on what needs to be done and when. Communication within your core team is critical. You should plan for daily, sometimes twice-daily calls, to make sure everyone knows what is going on in every region, and the response from customers, the media, and senior management.
3. Communicate the plan
You can assemble a great team and create a watertight plan but when it comes to an actual recall, it isn’t just your own team involved. Whether they be distributors, wholesalers or pharmacies, it is crucial for your business partners to understand their role in the plan. Companies throughout the supply chain should revisit the plan annually to ensure it takes current operations and personnel changes into consideration. The whole supply chain needs to know how and when you will communicate in the event of a recall and how the recall will be carried out. When a recall occurs, it should certainly not be the first time you are talking to them about your plans.
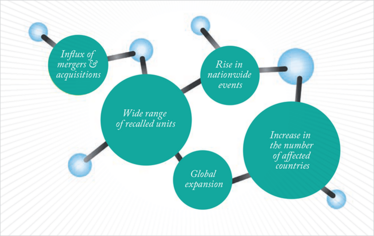
Figure 1: The increasing complexity of pharmaceutical recalls.
4. Be proactive, not reactive
When creating recall plans, a lot of energy is put into the launch – making sure that the communication is right and that the plan will be executed properly. Equally if not more important is to plan how you will respond during the recall. You need to know how quickly you can expect to see a response in different regions and track returns, questions and complaints in real time. This allows you to not only answer current questions but to anticipate upcoming questions.
5. Don’t underestimate social media
Don’t underestimate the power, both positive and negative, of social media. It can be a great friend in the midst of a recall, if you use it correctly and keep the message clear and concise. On the flipside, it can be critically damaging if the wrong information starts circulating. Confusion can spread very quickly and compound the crisis, so you will need to monitor and respond to social media traffic as it happens.
6. Test the plan
Planning alone doesn’t guarantee an effective recall – no orchestra performs perfectly by reading music sheets once! And so, like a well-practiced orchestra, you must rehearse your recall plan to ensure that all aspects have been considered. Recalls involve multiple phases and processes, so testing potential scenarios and the recall management team’s ability to respond will enable you to make adjustments where necessary. There are a variety of approaches to rehearsals. One of the most common is to simply ‘lock’ people in a room with a recall scenario, perhaps introducing a curveball halfway through to really test the strength of the team. Such exercises allow your team to respond to a real-life situation with critical, decisive thinking.
Taking things a logical step further, a mock recall (that is to say, actually sending out a mocked-up product) can test how reverse shipping would work in practice. I remember taking part in one simulation where, at a critical stage when testing had revealed that the recall needed to be expanded, it became apparent that no one on the team had access to the data needed to identify affected lots. This might seem like a trivial problem, but in an emergency you don’t want to lose valuable time waiting for a fix from the IT team. If you can’t pull the data quickly enough in a real-life scenario, the recall may have to be expanded – at considerable extra cost.
7. Look back
If you have a recall, it might be tempting to put it behind you as quickly as possible. But you would be missing an opportunity to learn a lot about your business and your supply chain. After every rehearsal or recall, meet with the team and make sure any lessons learned are shared with all relevant team members.
Having a good, well-rehearsed plan is clearly essential, but it is also imperative for manufacturers to stay informed on the safety standards of imported pharmaceuticals, as well as the distribution, handling and practices of global distribution networks. Having a deep understanding of current regional regulatory and legal mandates is of utmost importance, as multiple regionally targeted plans will be needed to adhere to local regulations.
Unfortunately, I think recalls are likely to become more complicated, not less; the increasing market demand for cheaper products is putting greater pressure on all manufacturers to spread supply chains even wider. But whatever the size or complexity of the recall, addressing the situation efficiently and calmly is key.
Companies that have practiced and prepared for the worst will be able to initiate a prompt, organized recall, which enables them to more quickly turn their attention to even more serious matters: correcting the problem that caused the recall in the first place.
As the Vice President of Recalls for Stericycle, Mike Rozembajgier has managed thousands of recalls across a wide variety of industries including pharmaceutical, medical device, consumer products and food and beverage. “After holding various management positions at Guidant Corporation (now Boston Scientific) and at Deloitte in their Strategic Consulting practice, I knew the constantly changing recall landscape presented a unique challenge that would keep the next step in my career fresh and exciting.” Mike received his BA in economics and computer applications from the University of Notre Dame, and an MBA from The Wharton School of Business.



















