
Inside the EMA: How to Accelerate Vaccine Rollout – Safely
Vaccine development for COVID-19 may have been rapid, but no one has scrimped on safety. Increased speed is the result of pooled resources, funds, and expertise.
“In the EU we have the important task as the European Medicines Agency and the enormous responsibility of evaluating the candidate vaccines. This vital work is needed to guarantee that when vaccines are used in the EU, we are sure that they are safe, of high quality and effective, and that their benefits outweigh any potential risk. We want the people of Europe to understand this work, the pride we take in doing this, and the enormous number of experts we bring together to make this happen, so people in the EU can trust the process, feel confident, and feel protected.”
- Emer Cooke, EMA Executive Director; speaking at the EMA Public stakeholder meeting on the development and authorization of safe and effective COVID-19 vaccines in the EU.
Transparency has been crucial throughout the COVID-19 pandemic – and the EMA has been keen to play its own role in this regard. In December 2020, the agency held a public meeting to explain how COVID-19 vaccines are being rapidly developed and evaluated – without cutting any corners in terms of safety. A number of experts from the agency gave presentations on the topic, including Marco Cavaleri, Head of Biological Threats and Vaccine Strategy, who outlined the main phases of vaccine development: pharmaceutical quality, non-clinical work, and clinical trials – emphasizing the extensive work and studies involved.
Early studies are conducted in the laboratory. In terms of pharmaceutical quality, the first steps include identifying the vaccine construct and technology, including, in the case of COVID-19 vaccines, what component of the SARS-CoV-2 virus will be incorporated into the vaccine to elicit the protective effect. As the candidate progresses, there will be more and more activity relating to pharmaceutical development. Key elements include:
- In vitro tests and assays to rapidly determine the biological activity the vaccine is exerting
- Characterization of all the components in the vaccine as well as determination of their purity
- Strategy to scale up small scale production to larger batches
- Incorporating good understanding of how the process can be controlled at each step of the manufacturing phase
- Tests to control manufacturing and ensure each batch of the vaccine is manufactured consistently and is of appropriate quality
- Starting stability tests as soon as possible to look at the stability of the vaccine at different temperatures
Extensive non-clinical work must be carried out before any vaccine – or therapeutic – moves to human studies. The immune response must be examined in animals, and toxicity tests need to check for safety problems, as well as problems relating to fertility or foetal development. “Another important area is conducting studies that are called the challenge studies, in which it is checked if animals after they receive the COVID-19 vaccines are protected from the disease when exposed to the virus deliberately. It is also important to make sure that the vaccination is not causing any paradoxical effects of worsening the disease as this is a signal that was seen in pre-clinical models with some investigational vaccines for other coronaviruses, such as SARS and MERS,” said Cavaleri. “Also, for some vaccines, it is very important to do some pre-clinical studies that could determine how the vaccine is spreading around the body; whether it’s not just staying around the injection site and the lymph node in the surroundings, but may be spreading to other parts and other organs.”

Clinical considerations
A large part of Cavaleri’s presentation was dedicated to clinical trials. He stressed that all trials must follow strict scientific and ethical rules, and must be approved by the competent authorities to safeguard the safety and rights of trial participants. The earliest human trials begin with a very limited number of volunteers, eventually moving to phase III trials, with tens of thousands of participants.
“Moving into COVID-19 vaccines specifically, the EMA has been engaging with developers and is giving scientific advice around what would be our requirements. We also recently published a reflection paper that summarizes our thinking around what would be required in these clinical trials,” said Cavaleri. “However, it is clear that, despite the studies being quite large and quite comprehensive, they will not be able to answer all the questions that you may have. In particular, what the studies might not be able to do at this stage is to give us sufficient information about the long-term protection that [the vaccines] can generate, and […] will not be necessarily informative with respect to the potential of the vaccine to block transmission of the virus in the community, which is, of course, a very important point from a public health perspective. And this will determine how to use the vaccine and what kind of coverage will be required in order to stop the transmission of the virus and put an end to the pandemic. For all of these very important questions, there is a need to conduct studies in the post-approval phase. This is what we’re already discussing with other players from a European perspective, and also with the companies, as it will be expected that they will have to conduct studies in this regard.”
Follow ups need to be done with trial participants around six weeks after the last dose of vaccine has been received; most side effects tend to occur 4-6 weeks after the vaccine. “However, these trials will last for one year or even longer in some cases, which potentially will give us invaluable information on the long-term protection that could be provided by the vaccines, and also whether some side effects may occur later on after vaccination,” said Cavaleri. “Whether participants in clinical trials can be kept on placebo long term after a vaccine is at a rise and made available in any specific country or location – that is something that, of course, we cannot predict and we might expect that it might be challenging and even not ethically viable. However, we are urging companies to try their best in order to make sure that these studies are continued in a rigorous way so that we have true evidence in the long run.”
Frontrunning COVID-19 vaccines have already demonstrated efficacy of 90 percent or more in large clinical trials and are being rolled out in some countries. According to Cavaleri, trials have been encouraged to include older adults. “When discussing with developers, we make it clear that we would like to see, ideally, one quarter of all participants above 65 years of age, as we know that age is a very important risk factor for mortality and morbidity due to COVID-19. Also, the inclusion of people with underlying conditions, such as cardiovascular disease or diabetes, has been strongly recommended, as we know that the risk of severe disease also in this population is highest. Some of the studies also include adolescents above the age of 16 years. However, for younger children, we expect that these studies will be conducted only once we have a good picture around the efficacy and safety of the vaccine in the adult population.”
He also added that there have been “genuine efforts” from drug companies to include ethnic minorities in COVID-19 vaccine clinical trials – but admitted that it is very difficult to be comprehensive.
Fast and furious
An important question that has been raised both inside and outside of the pharma industry is: Are COVID-19 vaccines moving too fast? Vaccine development typically takes years. So, how have we approved several vaccine options in various regions in less than 12 months?
In his presentation, Cavaleri pointed to Figure 1, which shows how non-pandemic vaccines are developed. Each stage occurs separately and, in many cases, there are intervals and pauses between each phase of development. Figure 2 shows what is occurring with COVID-19 vaccines. “With COVID-19, all these steps are still done – and in a rigorous way – but everything is much more compressed with many activities occurring in parallel or, with respect to the clinical phases, according to a seamless approach, where there is a continuum between the phase I, the phase II and the phase III trial,” said Cavaleri. “This has allowed something that has been unprecedented, which is seeing a vaccine develop in a matter of months instead of years. However, it’s important for me to stress that all this is happening without cutting any corners in the data generation. At the end of the day, we will have the same amount of evidence that we would have for any other vaccine developed in standard times. This has been possible because there has been an incredible effort by all the players and stakeholders to mobilize resources simultaneously and make sure by a number of agreements and collaboration to make sure that this vaccine could advance very rapidly. Also, they have sufficient funds to make sure that all these activities are carried on in really a very short time frame.”
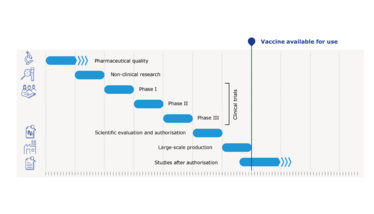
Figure 1: Standard vaccines compared with COVID-19 vaccines: an indicative timeline.
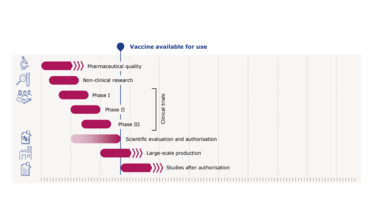
Figure 2: COVID-19 vaccine development timeline
Cavaleri added that the EMA has also contributed in terms of helping to accelerate the development process by establishing its COVID-19 task force to help facilitate continuous and rapid dialogue with developers and to give scientific advice to direct developers on regulatory requirements. The task force was set up in April 2020 to draw on the expertise of the European medicines regulatory network to ensure a fast and coordinated response to the pandemic.
“The other very important peculiarity that has happened here with COVID-19 is that manufacturers and sponsors have decided to start scaling up the manufacturing much before they were aware of whether their vaccine is successful in large clinical trials, so, in other words, starting scaling up at risk,” said Cavaleri. “This can be extremely important in making sure that once the vaccines are approved, there will be sufficient amount to be deployed in order to have an impact on the pandemic in the fastest possible way.”
Reviewing the evidence
There was also a presentation from Fergus Sweeney, Head of Clinical Studies and Manufacturing at the EMA, which focused on how exactly the EMA reviews its medicines – and how processes have been accelerated.
“Safety is paramount,” Sweeney emphasized. “Vaccines are given to a very large population; many, many of whom are healthy and certainly not yet suffering from COVID-19 in the large majority of cases, so [the vaccine] is to prevent that disease and, therefore, safety is very important. The benefits, of course, are very clear and we need to establish those and ensure that there is a high proportion of benefit to risk.”
He cited the “unprecedented” pooling of expertise in Europe to reduce timelines, as well as the agency’s rolling review process. “Rolling review enables us in a public health emergency to evaluate data as soon as available. As soon as a developer has the info or report on a particular study – be that pharmaceutical development, quality and manufacturing, non-clinical studies, or as they progress to clinical trial – they provide that to us. We can then evaluate that, ask questions, and evaluate the answers, so that, cumulatively, we are working our way through the dossier as the information becomes available. And the pace at which we can do that is driven by the availability of the information and the ability of the companies to respond to the questions that we raise,” said Sweeney. “Once all the quality, safety, and efficacy data are ready, the company then formally applies for the marketing application to the EMA. In all cases, this formal review and application will take place unless, for whatever reason, the clinical trial is at an earlier stage or some other development has prevented that from progressing.”
In a standard review, the developer will wait until they have completed all of the studies before proceeding with the regulatory evaluation. Steps are often very sequential – for example, they may not proceed with generating more regulatory data until the company has more confidence that it will be a successful medicine – and they only submit the marketing authorization when all of the data and studies are complete. Without the rolling review process, the agency would only just recently have started receiving full dossiers for COVID-19 vaccines – and the reviews would likely take time. This is not workable in a public emergency.
When the data has reached a sufficient point, the company can submit the full dossier for formal marketing authorization. “And because we’ve already gone through this period of rolling review, the outstanding work on our scientific experts to evaluate is much more limited and we are able to go through that process in a shorter time,” but without overall reducing the extent or rigor of our scientific evaluation or the necessary data that should be submitted,” said Sweeney.
The EMA is also using conditional marketing authorization processes to approve COVID-19 vaccines and treatments. “The benefits must clearly outweigh the risks and particularly with vaccines we need to be very sure that the safety is well understood and is at a very high level,” said Sweeney. “However, we can then make conditions on the marketing authorization that allow other data to be provided by the company after approval. For instance, the longer term follow up of people in clinical trials up to one year to show extent of and duration of protection from COVID-19, longer term safety information, but also studies, for instance in manufacturing, which may help to improve the storage conditions for the vaccines so that they can be made available on an easier basis.”
According to the EMA, Conditional Marketing Authorization has all the same standards and controls in place as the standard Marketing Authorization process (see Figure 3). Sweeney said, “Vaccination will be carried out in many, many people, and we really need to have a high standard of assessment before that takes place but also a very rigorous control framework with which to follow those vaccines as they are used on the market. This is really important to give our citizens confidence in the safety and efficacy of the vaccines – because the willingness of all of us to participate in these vaccination campaigns is also very, very important, and our confidence in those and the confidence of citizens is paramount.”
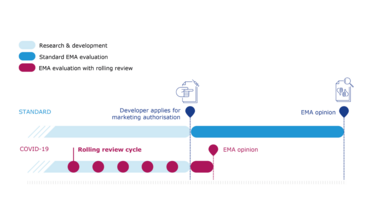
Figure 3: The rolling review of COVID-19 vaccines
COVID-19 is a public health emergency, so more data on vaccines currently approved under Conditional marketing Authorization are expected. The EMA has mandated its own studies and monitoring, and public health authorities will also be carrying our similar work. Sweeney said, “There is stringent scientific evaluation by EMA on the quality, safety, and efficacy, with an unprecedented pooling of EU expertise from across the network, rolling review of data as it becomes available to enable fast approval, and conditional marketing approval providing strong safeguards for the authorization, and for the supervision of the product and the evaluation of new data as it becomes available.”
The full presentations and recordings are available on the EMA website

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















