Land of Opportunity
Biosimilars had their big break in the US in 2015, but three years later biosimilars are still struggling to make a mark.
Since its founding in 2015, the Biosimilars Council – a division of the Association for Accessible Medicines – has worked to ensure a positive, reimbursement, political and policy environment for biosimilar products, and educate the public and patients about the safety and effectiveness of biosimilars. We spoke with Craig Burton, Vice President of Policy at the Association for Accessible Medicines to learn more about the thorny path for biosimilars in the US.
What are the biggest challenges and hurdles to biosimilar adoption in the US?
Biosimilars manufacturers face typical pharmaceutical development challenges related to science, regulation and production. Notably, this includes a series of artificial hurdles intended to thwart competition, including schemes to limit access to samples needed for development and the creation of “patent thickets” designed to delay competition. Even after a biosimilar makes it onto the market, it will likely face anti-competitive tactics designed to limit its ability to garner significant market share. The high-profile Pfizer versus J&J Remicade court case highlights the use of exclusionary contracting to limit biosimilar formulary placement (1). This restricts the ability of the biosimilar to get into the hands of patients and undermines the incentive to develop these products in the first place.
Fortunately, since the introduction of the first biosimilar in 2015, there have been positive developments in Medicare reimbursement to provide consistent and predictable reimbursement for biosimilar manufacturers. In particular, establishing independent coding and reimbursement in outpatient settings for biosimilars in Medicare Part B and putting biosimilars on equal footing with their brand counterparts in Medicare Part D gives biosimilar manufacturers greater certainty when planning their portfolios.
At the Biosimilars Council, we believe that policymakers need to closely observe the progress of the biosimilars market to ensure policies are in place that effectively maximize competition and incentivize biosimilar development.
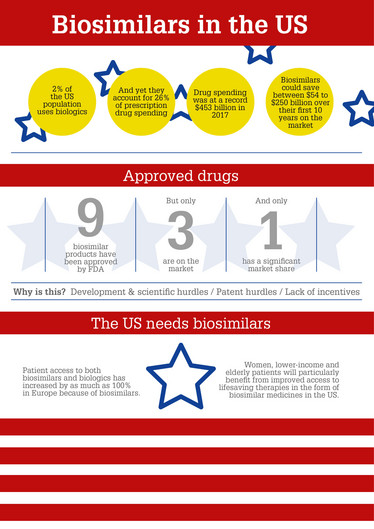
The US has seen a lot of lawsuits and “patent dances” regarding biosimilars’ how are these affecting the industry?
The “patent dance” issue has largely been resolved with the Supreme Court’s decision in the Sandoz Inc. versus Amgen Inc. case. The court ruled biosimilar applicants cannot be forced under federal law to engage in the Biologics Price Competition and Innovation (BPCIA) Act’s information exchange and that they need not await FDA approval before providing the statutorily required notice of commercial marketing. While this provides significant clarity to the “patent dance” process, the BPCIA expressly provides that a reference product manufacturer may seek to prevent the biosimilar applicant from making or selling its product until the court resolves patent validity, enforceability, and infringement after it receives the notice of commercial marketing.
As you would expect, the intellectual property that relate to biological products are significantly more complex than small molecule generics. To that end, protracted litigation between biosimilar manufacturers and brand biologic manufacturers following commencement of the “patent dance” continues to hinder the entrance of several of the FDA-approved biosimilars.
The Biosimilars Council supports avenues outside of the court system that streamline this process, including administrative processes under the jurisdiction of the US Patent and Trademark Office, that serve as efficient tools for biosimilar manufacturers seeking to invalidate frivolous patents. We also support contractual agreements between brand and biosimilar manufacturers that bypass the litigation process and provide certainty to biosimilar manufacturers as to when they can enter the market. Several manufacturers of currently FDA-approved biosimilars have entered into such agreements with their brand counterparts.
What is the FDA doing to improve biosimilars development and access?
The BPCIA established and empowered FDA to implement an abbreviated pathway for biosimilar review and approval. While the FDA has made significant progress in the eight years since the BPCIA was enacted, there are a number of unresolved issues that will affect the viability of biosimilar development, including those related to the naming and labelling of biosimilar products, as well as those related to interchangeability and extrapolation of indications.
FDA Commissioner Scott Gottlieb has affirmed the agency’s commitment to increasing the efficiency of the biosimilars pathway by developing a “Biosimilars Action Plan” that will help address some of the current regulatory uncertainty. According to the Commissioner, FDA is working on “about a dozen policies” that will include “new tools and information resources that can assist biosimilar sponsors in developing high quality biosimilar and interchangeable products using state of the art analytical techniques. These tools can support…more efficient biosimilar development programs without compromising on our scientific rigor.”
Additionally, as part of the new User Fee Agreements passed last year, biosimilar applicants will be able to better communicate with the agency to put forward the best application possible, and provide the necessary evidence as early as possible.
What changes would you like to see in the industry to better incentivize the development of new biosimilars?
The continued development and implementation of policies that provide consistent and predictable reimbursement for biosimilar products, addressing anti-competitive behavior by brand pharmaceutical firms and resolving the regulatory uncertainty and market entry challenges we’ve discussed.
Biosimilars mean new access to life-saving biosimilar medicines for millions of patients, while also promising savings for patients and the US healthcare system overall. FDA Commissioner Gottlieb highlighted the importance of fostering the development of a strong biosimilars market at America’s Health Insurance Plans’ (AHIP) National Health Policy Conference, saying, “The public health benefits of a robust, competitive market for biosimilars are impossible for us to ignore. Strong market incentives are critical to future biosimilar development in the same way these incentives are key for the development of innovator drugs and biologics.”
The promise that biosimilars hold can only be realized if policies are put into place that promote the development of a robust market.
- Reuters, “Pfizer files suit against J&J over Remicade contracts,” (2017). Available at: reut.rs/2Hurmgc. Accessed April 27, 2018.

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















