A Clearer (Chemical) Picture
How a niche high-resolution microscopy technique could power drug discovery and development

From left to right, Professor Giovanni Costantini and Professor Gabriele Sosso
Researchers using a combination of high-resolution scanning tunneling microscopy (HR-STM) and first-principles simulations have been able pinpoint the location of atoms and identify halogen bonding (1) – and they believe that such precision can be exploited as a tool in pharmaceutical drug development. Here, Giovanni Costantini, Professor of Physical Chemistry at the University of Warwick and one of the project’s lead researchers, describes the capabilities of HR-STM – and how it fits into pharma’s analytical toolbox.
What is STM?
STM is a wonderful experimental technique whose invention is often associated with the birth of nanoscience and nanotechnology. In fact, the 1986 Nobel Prize for Physics was partially awarded to Gerd Binnig and Heinrich Rohrer for their design of the scanning tunneling microscope, indicating how important the technique is for fundamental science. STM exploits the minute current that flows between a very sharp metallic tip and a surface when separated by a distance of about 1 nm or less (this is a quantum mechanical effect called quantum tunneling – from which the microscope derives its name). STM allows the location of molecules to be mapped at a resolution typically below 1 nanometer.
What challenges are associated with the use of STM?
When imaging molecules using STM, the intensity of the current that flows from the needle tip to the surface is often rather uniform across the molecule itself, and while individual molecules are visible, it is generally not possible to resolve their internal structure. And that makes it difficult to determine the precise chemical structure of an unknown molecule or to ascertain the way molecules interact with one another.
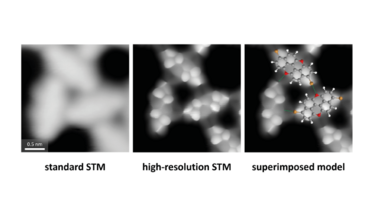
Microscope images of the molecules progressing in clarity
What did your research show?
In high-resolution STM, an individual carbon monoxide molecule is picked up with an STM tip. This process makes the needle “sharper” and more chemically defined. We compared the imaging capabilities of HR-STM with standard STM by testing the techniques on brominated polycyclic aromatic molecules that self-assembled on a gold surface. This comparative study showed us that STM was unable to determine whether the intermolecular interactions between the molecules we studied were hydrogen or halogen bonds. But because HR-STM allowed us to bring the needle tip extremely close to molecules, we were able to locate carbon rings within the molecule and identify that halogen bonds were responsible for its assembly. Notably, this feat could only be achieved in a controlled manner when the microscope was operated at temperatures close to −269 °C. Simply put, our experiments demonstrate that it would not have been possible to capture this level of detail using standard STM alone as the lower resolution images obtained were compatible with both intermolecular hydrogen and halogen bonds.
We also determined that the samples we analyzed contained small amounts of impurities derived from chemical synthesis and often present in such small amounts that are below the sensitivity of standard analytical techniques. These impurities had indeed escaped the scrutiny of conventional spectroscopic characterization techniques, which were essentially blind to them. We were given a clear indication that HR-STM has the potential to be used as an analytical tool for chemical structure determination.
Where does HR-STM fit into drug development?
Our project was a fundamental study, but we hope it might have value for researchers attempting to understand (bio)molecular recognitionand for those interested in designing novel materials and functional molecular entities, possibly even for pharmaceuticals drugs. In trying to determine whether hydrogen or halogen bonds were present between molecules, we have also shed light on a relatively widespread but so far comparatively little studied intermolecular interaction, halogen bonding.
Halogen bonding has recently been found to play a significant role in the structure of biological macromolecules. Yet, its use to control intermolecular interactions is often considered as unconventional and its potential to improve pharmaceutical products has only begun to attract attention from the scientific community in recent years. In fact, most medicinal chemistry research has focused on the role of hydrogen bonds because of their ubiquity in biochemical and material sciences. But research now shows that halogenated macromolecules can be used as drugs, drawing on the relevance of halogen bonding in the folding of protein-ligand and DNA structures, as well as substrate specificity. Though it is true that halogenated nucleic acids are already used as chemotherapeutic agents, more needs to be done to take advantage of what these types of molecules have to offer and to engineer the next generation of molecular systems for drug design.
What's next for this research?
We plan to extend the application of HR-STM and of a similar related technique – non-contact atomic force microscopy – to study other types of "unconventional" intermolecular interactions and to untangle the interplay between halogen and hydrogen bonding in the assembly of small molecules of or biochemical applications and materials science.
We also plan to use these high-resolution molecular imaging techniques to determine the structure of unknown molecules – the cases where conventional analytical techniques have failed. The long-term goal? To take what, at present, is still a niche experimental technique, developed and used in the rather limited research areas of surface- and nano-science, and to transform it into a new analytical tool for the much wider fields of synthetic, pharmaceutical, and biological chemistry.
- J Lawrence et al., “Combining high-resolution scanning tunnelling microscopy and first-principles simulations to identify halogen bonding”, Nat Commun, 11, 2103 (2020).
After finishing my degree, I envisioned a career in science communications. However, life took an unexpected turn and I ended up teaching abroad. Though the experience was amazing and I learned a great deal from it, I jumped at the opportunity to work for Texere. I'm excited to see where this new journey takes me!



















