Accelerate liquid formulation development for protein biopharmaceuticals
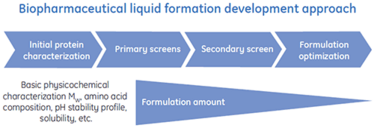
The work presented here illustrates how Differential Scanning Calorimetry (DSC) is used in the early stages of protein characterization to rapidly provide critical data about protein stability that can be used as a guide to support and accelerate liquid formulation.
Introduction
Monoclonal antibody development projects exist for a wide variety clinical indications (1,2). Recombinant proteins of commercial interest, including monoclonal antibodies, need additional properties beyond their biological activity to enable their development into successful biopharmaceuticals. Particularly, they need to be resistant to chemical degradation and be physically stable in a given environment, without any tendency to aggregate (3,4). Furthermore, they need a favorable serum half-life and should exhibit no, or very little, immunogenic potential (5).
Apart from being more expensive to produce than liquid formulations, a lyophilized drug product (DP) must be reconstituted by the physician, taking up to 10 to 20 minutes before parenteral administration to the patient. The main driving force pushing the biopharmaceutical industry to focus resources on developing liquid formulations instead of conventional lyophilized DPs is simplified administration. However, there are some technical issues to be overcome with regard to liquid formulations. The main challenge is to keep the protein biopharmaceutical stable in a liquid formulation by maximizing the physical stability and minimizing chemical degradation. It is especially challenging to develop biopharmaceuticals for subcutaneous administration because here the liquid formulation should have as high concentration of protein as possible to compensate for the limitations imposed by possible injection volume, 1.0 to 1.5 ml (6).