
Balancing the Cost of Success and Failure
Publishing negative results might not flatter – but it does matter.
Late on in my career at GlaxoSmithKline, my colleagues and I published a paper (1). It could have been groundbreaking. In some ways it was. We were seeking a better way to treat wet advanced macular degeneration (AMD) – one that would obviate much of the burden of monthly clinic visits for intravitreal injections of anti-VEGF therapy (anti-vascular endothelial growth factor therapy – used to reduce new blood vessel growth or swelling in the eye) that we see today. Despite solving many problems along the way, ultimately, the project failed – but we should all learn from its failure. Let me tell you my story.
Discovering ophthalmology
It started back in 2007, when I first worked in the field of ophthalmology. GSK’s head of research at the time was Tachi Yamada, and he was interested in the gene therapy area. He knew one of the biggest names in that field – Jim Wilson at the University of Pennsylvania – and he enabled GSK to access some of the Wilson group’s novel adeno-associated virus (AAV) vectors. We started working with Jim’s group and also a number of researchers from the University College London Institute of Ophthalmology in London looking at gene therapy approaches to ocular disease. The opportunities were great – here was an organ where you could actually see the effects of what you were doing! We also started re-profiling existing GSK assets and considering ophthalmic applications for them – this was a therapy area that had great promise! I started going to the meetings of the Association for Research in Visions and Ophthalmology to start trying to understand what kind of problems were out there in ophthalmology: scientifically, clinically, and everything else that we might need to deal with when building an ophthalmic franchise. It was apparent even then (ranibizumab had been approved in the US less than a year before for the treatment of wet AMD) that the large number of clinic visits and intravitreal anti-VEGF injections – one a month, going forward for as long as the drug continued to work – was going to be the big issue, in addition to the hefty cost of the drugs themselves.
Combining drug with delivery platform
Around that time, GSK acquired a company called Domantis that worked on domain antibodies and antibody fragments. As part of that, we acquired certain relatively small anti-VEGF molecules – certainly smaller than ranibizumab. Here was an opportunity to play with drug delivery – i.e. to pack a lot of drug in to a sustained-release vehicle and build a long-acting injectable anti-VEGF. So we presented that idea to the GSK equivalent of Dragon’s Den – an internal poster presentation and competition session called the Goldfish Awards. We didn’t win – but what we presented generated enough interest within the newly formed GSK Ophthalmology group that they thought it that was a good idea to pick up. We started reviewing drug delivery options, and came across a Dutch company called Octoplus N.V. (now part of the Dr. Reddy’s franchise) who had an aqueous hydrogel drug delivery platform (Figure 1) that not only managed to keep the proteins active for a long time, but also released them with pretty much first order kinetics – i.e. with minimal “burst” release – over a long period. They hadn’t really performed any studies in the eye, so we moved forward together.
Even then, there were stumbling blocks. Our original candidate molecule just wasn’t potent enough, so the big challenge was rebuilding the molecule to make it a more potent VEGF inhibitor. What we ended up making (Figure 2) was at least as potent as the most potent anti-VEGF available on the market today, aflibercept (2). We then had to work to find and evaluate a polymer that could keep the protein intact in the distinctly “wet” environment of the eye and release therapeutic levels of it over a 6–12 month period. That was no easy task: the principal technical challenge was to load enough protein material from the antibody fragment into the microparticle itself – you needed to get liquid protein concentrations up to >200 mg/mL (a huge amount) to enable the release of sufficient quantities to be effective for at least 6 months. But we did it.
Progressing through the preclinical steps
The next step was preclinical in vivo experiments. We did our first studies in rabbits, as it is the model of choice for studies of ocular delivery. The use of rabbit eyes are not without issue; though the intravitreal volume is relatively large at 1.3 ml, it is still smaller than man (4.5 ml), but with a huge lens, so you need to make sure that injection avoids the lens – and then there were issues with immune responses. Our antibody fragment was a humanized protein: the rabbit’s immune system kicked in and generated anti-drug antibodies (ADA) responses post-dose at high frequency, which blocked detection of the released anti-VEGF and made it challenging to interpret the results. Nevertheless, we gathered together enough data to demonstrate that substantial levels of active anti-VEGF molecule were present in the rabbit vitreous at six months post-dose and to justify progressing to the non-human primate (NHP) model. The cynomologous monkey is far closer than rabbits to humans in terms of ocular anatomy and function – and it also seemed likely that the closer similarity to human would help reduce the negative impact and frequency of ADA responses; enabling simpler detection and interpretation of the pharmacokinetics of the released molecule. It turned out that these successful and very expensive experiments both validated many aspects of the approach but also ended the project…
We’d found that in vitro, we could release effective doses of anti-VEGF molecule from the microparticle/ hydrogel, PolyActive, platform over a 12-month period, and in vivo, this translated to at least six months’ worth of effective levels of anti-VEGF released in both the rabbit and NHP experiments. We used the NHP laser choroidal neovascularization (CNV) model (the pre-clinical model of wet AMD used to validate ranibizumab prior to the clinic) to test how successfully our therapy managed to suppress the production of laser-induced leaky new blood vessels: we’d dose the eyes, wait 4–6 months, and challenge the eye with the laser – and found that we still got good protection even 6 months out. In that respect, moving forwards to a clinical trial looked promising. But there were three major challenges that prevented us from doing so – and these represent crucial lessons for any other research group that is trying similar intravitreal particle-based drug delivery systems.
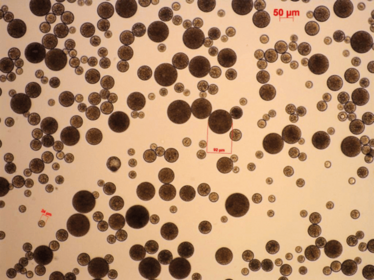
Figure 1. PolyActive hydrogel microparticles. Adapted from (1).
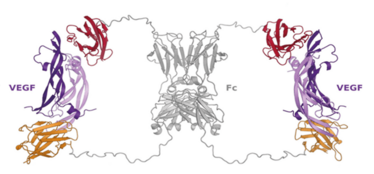
Figure 2. Proposed structure of the dual domain antibody in complex with two VEGF molecules. Adapted from (2).
Key Learnings
- Collectively, companies and research institutions have invested millions trying to develop intravitreally administered, extended-release anti-VEGF agents for the treatment of retinal neovascular disease that can act for as long as 6 months.
- GSK, in collaboration with OctoPlus N.V., developed a novel potent anti VEGF molecule and hydrogel microparticle combination that almost fitted the bill – and was close to a clinical trial. Had it worked, it would have been a paradigm changer.
- One of the issues that led to the project’s termination was caused by fundamental and poorly understood aspects of primate accommodation, which led to microparticle migration into the anterior chamber.
- The issues highlighted in this research are relevant for others pursuing the use of particulate injectables for intravitreal ocular delivery, some of whom are not easily able to afford the key experiments in the primate eye needed to de-risk likely similar issues in man.
Three big challenges
The first hurdle was ocular inflammation: we were seeing it in the NHP eyes, as well as the rabbits. Although both protein and microparticles were prepared at high quality and were shown to have extremely low levels of endotoxin, they were still research-grade materials, i.e., not prepared at GMP grade purity. So it might have been possible to reduce the degree of inflammation by improving the quality of what we were administering, unless the inflammation was solely driven by the particulate nature. But these weren’t the only challenges. The second issue was a lack of degradation of the polymer at the same rate as the release of the molecule. The polymer was predicted to last for 6–9 months, based on experiments where similar PolyActive implants had been placed subcutaneously in rats – but when we looked at the PolyActive material in the NHP eye, it was still there at 6 months, 9 months… and it was only really 12 months after implantation before we started to see any major en masse reduction and degradation. That meant it would be very difficult to re-dose – the accumulation of material in the eye would start to become a problem after only a few doses. But the third and biggest problem was related to the microparticles themselves. They would travel from the vitreous into the anterior chamber. These three issues combined lead to termination of the project. We were quite surprised by the latter observation – we hadn’t seen anything like that in our rabbit studies, and it seemed to be driven by the primate (and presumably human) eye’s process of lens accommodation-disaccommodation (3). It seems that ciliary muscle-driven lens movement causes fluid to flow between the vitreous and anterior chamber, and the particles get disturbed and caught up in it. And so, despite some great technical achievements along the way – developing a potent anti-VEGF antibody fragment, and being able to concentrate, load and deliver this biologic over a long period with a novel drug delivery vehicle – we fell at this last hurdle.
Being open
Why am I talking about our work, both the successes and its ultimate failure? I strongly believe that negative results, especially those that have such a strong bearing on the future of a field should be published (4). Anyone evaluating a similar drug-delivery method needs to be aware of our work – GSK was not alone in working on this approach. There are still biotech companies developing particle-based drug delivery approaches for intravitreal injection who have not performed NHP studies and are either unaware of our findings or are reluctant to accept the full consequences of them, as it might negatively influence their share price. Also, how many biotech companies and academic groups are receiving funding from research councils or companies to fund costly studies – only to repeat our findings? A huge combined investment has likely already been made with this type of approach by GSK, together with other pharma and biotech companies. Although our project didn’t work out, there were positive aspects from our study. We clearly demonstrated that hydrogel systems can keep anti-VEGF protein molecules stable and active, and can enable them to be released for over 6 months in the eye at effective doses to treat wet AMD. I hope that others will learn of and from our experience – and not feel the need to cover old ground.
The big questions I’m left with are: how can others build from the positive aspects of our findings and address the remaining issues? Can those working on particulates really take heed of the full message and switch funding and research activities to concentrate on generating similar data to ours with temperature-sensitive solidifying erodible gels? If others with a negative data story are reluctant to share knowledge with the field perhaps they should reconsider and think of what other medical research could have been done with the money others spend repeating their mistakes. The answer to that latter question is the true cost of failure.
Ian Catchpole is a GSK Fellow, Cell & Gene Therapy, and is based in their Stevenage campus in Hertfordshire, UK.
This article was originally published in The Ophthalmologist, a sister publication to The Medicine Maker –
www.theophthalmologist.com.
- P Adamson et al., “Single ocular injection of a sustained-release anti-VEGF delivers 6 months pharmacokinetics and efficacy in a primate laser CNV model”, J Control Release, 244, 1–13 (2016). PMID: 27810558.
- A Walker et al., “Novel Interaction Mechanism of a Domain Antibody-based Inhibitor of Human Vascular Endothelial Growth Factor with Greater Potency than Ranibizumab and Bevacizumab and Improved Capacity over Aflibercept”, J Biol Chem, 291, 5500–5511 (2016). PMID: 26728464.
- MA Croft et al., “Mechanism of accommodation: new findings and the implications for presbyopia”, IOVS, 56 (2015) ARVO E-Abstract 3568. Available at: biy.ly/macroft.
- K Park, “Ocular microparticle formulations for 6-month delivery of anti-VEGF”, J Control Release, 244, 136 (2016). PMID: 27964806.
Ian Catchpole is a GSK Fellow, Cell & Gene Therapy, and is based in their Stevenage campus in Hertfordshire, UK.



















