Better Bioanalytics = Better Bioprocesses
A thorough understanding of your process and how it impacts your product’s critical quality attributes is essential for decision-making, development and scale up. Are you up-to-date with new analytical platforms that can give you the edge?

Never before have biopharma companies been under more pressure to deliver products to market faster, while maintaining increasingly high standards of quality – particularly when it comes to biosimilars, where the analytical burden is even higher. Are more advanced bioprocess analytics the key to success?
Fredrik Sundberg from GE Healthcare believes so. Sundberg has spent his entire career focusing on process development, starting at Pharmacia & Upjohn in 1994, which is today part of Pfizer. Since then, he has worked in a number of roles connected to biosensing and enjoys driving collaborations within the bio/pharma industry around analytical and validation strategies. Today, he is Global Director for Strategic Customer Relations and Market Development at GE Healthcare, but also consults with health authorities to improve the safety, quality and productivity of current protein analysis workflows. Here, Sundberg explains how bioprocess analytics can facilitate development – and time to market.
Where do analytical tools fit into bioprocess development and control?
The goals of process development are to obtain high product purity and (hopefully) good process economy, while ensuring that important drug properties are maintained and that the process itself is well-controlled and validated, and with low impurity levels. Achieving these desired outcomes is much easier if you have an in-depth understanding of how both upstream and downstream processes can affect the critical quality attributes (CQAs) of your product. This knowledge is important not only for development and scale up, but also for future manufacturing. For example, during a drug’s lifecycle, there can be a number of manufacturing changes, such as alterations in upstream conditions of cell line and media components, or changes in purification methods to improve purity and yield.
When working with biopharmaceuticals, it is important to maintain a well-characterized product. It is widely accepted that biopharmaceuticals are far more complex than defined chemical drugs, so analytical tools play an extremely important role in terms of developing an effective control strategy for the bioprocess and developing a more efficient and reproducible process overall. A variety of different tools can be used to achieve this. Typical analytical tools include mass spectrometry (MS), high-performance liquid chromatography (HPLC), LC-MS, isoelectric focusing, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), western blotting, ELISA, and surface plasmon resonance (SPR), as well as various bioassays.
How have analytical technologies advanced over recent years?
There have been vast improvements in the productivity of analytical technologies, such as better integration into workflows and improved software. I’m also seeing the introduction of more information-rich technologies and higher resolutions that give greater, in-depth information about how process parameters can affect CQAs. For example, advanced biophysical methods (traditionally only used in research to understand the mechanism of action of a product or to characterize CQAs in early proof of concept) are now moving downstream and becoming part of process analytics and release analytics within quality control. Technology companies are also really pushing the limits in terms of sensitivity and throughput, and people are no longer talking about time to result, but about time to decision – how quickly can we decide if a drug candidate is suitable? And how quickly can we release a batch?
The most effective technologies available today for physicochemical characterization and structural determination are different types of HPLC, MS and LC-MS, as well as differential scanning calorimetry approaches. In terms of functional characterization, label-free technologies, such as SPR-based biosensors, have made a significant contribution over the past few years, providing higher-throughput assays in multiplexing formats with unparalleled sensitivity. It’s now possible to use these assays throughout the entire workflow, from discovery to early development and all the way through to final commercial release testing. Quality assurance and quality control are also going through a transformation thanks to the implementation of “surrogate” potency assays – binding assays that complement or replace cell-based assays. Binding assays can be used if the mechanism of action of the drug product is binding-blocking and/or can be replicated with ligand binding. These automatable, label-free assays are less resource intensive than cell-based assays or ELISA, and offer higher precision and accuracy with less artifacts, and shorter development time. A number of large biopharma companies are now adopting these assays and there are several FDA- and EMA-approved SPR potency assays on the market.
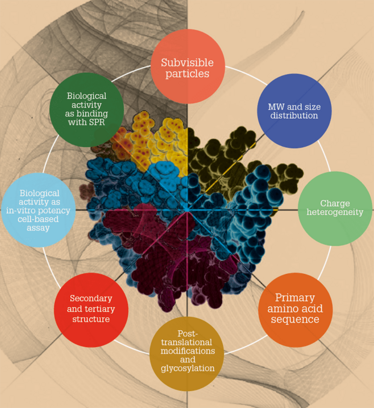
A number of factors need to be analyzed and controlled when developing a biosimilar.
Given that so many different techniques and technologies are available, it is possible and advisable to use an orthogonal approach when it comes to understanding your molecule – something that regulators also recommend. Orthogonal modes of analysis allow you to establish, with confidence, that your process is under control and that you are really measuring what you think you are measuring. For example, you may need to measure binding using the SPR-based platform first, and then later use a different technique, for looking at the affinity or impurity profile of a molecule for its target receptor. Having a tool-box with different analytical technologies at hand can be very useful when characterizing complex biological products.
Do biosimilars pose an even greater analytical challenge?
Biosimilars are a growing section of the market (thanks to recent approvals from the FDA) and analytics are used in all phases of their development, from early selection to proof-of-similarity. One of the main challenges is that the biosimilar must be nearly identical to the reference medicine (notwithstanding minor differences in clinically inactive components), which requires extensive physicochemical and biological characterization. In fact, the analytical burden for biosimilars can be even higher than for normal therapeutics. Failing to demonstrate similarity can come with high costs – since more extensive clinical trials may be required to get the product onto the market.
Guidance documents from regulatory health authorities, such as the FDA and EMA, emphasize the importance of extensive analytical characterization in showing similarity between a biosimilar and its reference medicine – often recommending the use of a variety of techniques to develop a comprehensive assessment of protein structure and function. As such, developers of biosimilars must put together a toolbox of state-of-the-art technologies. Companies like Sandoz and Celltrion, which have both recently received FDA approvals for biosimilars, have had great success in using real-time, label-free biophysical analytics as a platform approach, where multiple key applications were evaluated with one technology (product and process support; evaluating target and receptor binding; measuring immunogenicity and specificity).
Another significant challenge with biosmilars – as well as other biological products – is standardizing Fc receptor binding. Fc receptors can be activating, or inhibitory, or without any effect in antibody-dependent cell-mediated cytotoxicity (ADCC), and complement dependent cytotoxicity (CDC), as well as playing a key role in the mechanism of action for several anticancer antibodies. Finding new ways of studying these, and monitoring them from a manufacturing and comparability perspective, as well as conducting quality control,
is challenging.
Where else are advanced bioanalytical technology platforms being adopted?
The vaccine industry has traditionally been more conservative than the biopharma industry in terms of implementing novel technologies; however, with a number of pandemics occurring in recent years, the industry has been receiving more funding for research, development and manufacturing – and is now starting to implement advanced technologies. Some of the toughest analytical challenges can be found in vaccine development and there is a huge demand for better in-vitro characterization analytics, particularly in routine testing. Here biophysical methods, such as SPR, have an increasingly important role. For example, throughout the development process, SPR interaction data can contribute to detailed understanding of a vaccine’s properties and characteristics, as well as potency. High-quality analytical tools with higher precision and throughput are required to measure the recovery, yield, and purity of modern vaccines, such as polio, influenza, and virus-like particles (VLPs). SPR biosensors, such as those supplied GE’s Biacore™ portfolio, have been successfully employed to accelerate optimization of process parameters and to perform batch release tests. Gardasil™ (Merck Sharp & Dohme) and Cervarix™ (GlaxoSmithKline) are prophylactic VLP-based vaccines developed for the prevention of cervical cancer, and in both cases, the manufacturers use SPR systems to detect antigen availability in the VLPs.
Analytical technologies and sensors can also be very useful for next-generation biologics, such as bi-specifics (dual specificity for two targets) and antibody-drug conjugates (combining antibody specificity and the effectiveness of toxic small molecules), which are both popular avenues of new drug development today. Characterizing these highly complex products and their interactions, however, is extremely challenging.
What best practice tips can you offer for effective analysis?
Always focus on the quality attributes of the molecule and then pick the best fit-for-purpose analytical method. Most importantly, don’t be afraid to choose new, more advanced technologies or techniques that you may not be familiar with. One of the great benefits of technology today is that there is a huge emphasis on usability, which means that you don’t have to be an expert to use the best analytical equipment in the field. These new technologies and smart software tools can really speed up the decision making process. Regulatory authorities strongly recommend the use of “state-of-the-art” technologies, as described in various guidance documents. When applicable, using a direct binding assay to assess potency with real-time detection and precision instead of a time-consuming bioassay with high variation can significantly improve both productivity and control. A platform approach with a “QC-friendly” analytical technology delivering stability-indicating data in R&D with higher precision should also be considered for later-stage applications, such as in-process and quality control.
Additional information can also have an impact. For example, the use of binding kinetics measurements to profile binding to support in-process control in combination with the introduction of novel comparability software tools for “finger-printing” can provide unique insights into the effect of the critical process parameters on CQAs, and can be applied to all complex biologics. Such information-rich analytical tools have the potential to improve not only the probability of success at early-stage development, but also long-term comparability programs and late-stage potency assessment, which could ultimately result in a much leaner and more productive process for establishing well-characterized biological products.
But remember, no single technology can solve all your problems so you need to develop a toolbox. Different analytics give answers to different questions about a molecule – and sometimes an orthogonal approach is the only way to gain a comprehensive answer. Biological molecules are complex (and always will be), but new technologies are able to deliver effective bioanalytics, giving manufacturers the insight to improve their process and product – and to make the right decisions early on.
Fredrik Sundberg is the Global Director for Strategic Customer Relations and Market Development at GE Healthcare.



















