Crystal Clear Predictions
A new model for cocrystal formation could be a powerful tool for formulation scientists – no crystal structures required, it’s all about the surface contacts.
Cocrystals – two or more molecular entities combined in a homogenous crystalline structure – have recently become attractive targets for the pharmaceutical industry, as cocrystallization can have a positive effect on the properties of solid dosage forms. However, predicting which potential coformers will successfully cocrystallize with a given active pharmaceutical ingredient (API) has proved difficult. Here, we describe a new computational method that does not require any knowledge or prediction of three-dimensional crystal structures, making it fast enough to virtually screen very large libraries of compounds to successfully identify new API cocrystals.

Hunting cocrystals
Formulation of a drug as a cocrystal can change the bioavailability, dissolution rate, physical and chemical stability, compressibility and hygroscopicity, and new multicomponent solid forms offer the possibility of releasing an API without infringing the originator’s patent (1). Cocrystals may also occur as multiple polymorphs, suggesting additional options to modify properties, increased patent protection and improved formulations. In 2012, the FDA issued a set of guidelines to regulate the use of pharmaceutical cocrystals and concluded that a cocrystal should be considered as a drug product intermediate and not as a new API (2). In July 2014, the European Medicines Agency (EMA) published a reflection on the use of cocrystals of active substances in medicinal products and determined that cocrystals were eligible for generic applications in the same way as salts (3).
Cocrystals have a number of advantages over salts. For example, there are a large number of potential coformers contained in the GRAS (Generally Regarded as Safe) and EAFUS (Everything Added to Food in the United States) lists published by the FDA. In contrast to salts, the formation of a cocrystal does not rely on ionization, so it is not limited to APIs containing acidic or basic functional groups. This means that the structure space of potential formulations with improved properties is vast. However, this spectacular potential presents the experimental scientist with a difficult challenge: how can we navigate such a vast molecular landscape?
The crystal maze
Historically, a knowledge-based approach has been used to select appropriate cocrystal coformers, exploiting experimental data contained in the Cambridge Crystallographic Database. This approach to structure-based cocrystal design uses a statistical analysis of functional group interactions in the solid state to identify supramolecular synthons. Supramolecular synthons have their origins in the lock and key principle described by Emil Fischer in 1894 (4) and were defined by Gautam Desiraju as “structural units within supermolecules which can be formed and/or assembled by known or conceivable synthetic operations involving intermolecular interactions” (5).
In the 1980s, Margaret Etter deduced a set of empirical rules, which many chemists have used to predict the formation of multicomponent crystals (6). While the synthon approach focuses on structure, Etter’s rules provide a recipe for predicting which interactions are most likely to occur in the solid state based on energetics. The preliminary steps in the design of multicomponent crystal phases has traditionally incorporated both strategies, identifying reliable supramolecular synthons and using empirical data to estimate an energetic hierarchy. However, cocrystal prediction is hampered by variations in cocrystal stoichiometry, the presence of secondary weaker intermolecular interactions and the possibility of additional components, such as solvent molecules. Reliable prediction of whether a specific API and coformer will actually cocrystallize remains a challenge, and as a result, cocrystal preparation is still mainly conducted through trial-and-error experimental screening.
Put simply, we expect a cocrystal to form if it is thermodynamically more stable than its components. Thus, understanding the energetics of non-covalent interactions is essential for predicting whether a particular combination will form a cocrystal. In principle, the energetics of cocrystal formation could be calculated directly using ab initio methods (7). However, one of the biggest limitations in the development of computational methods for predicting cocrystal formation is that a crystal structure must be known to calculate the lattice energy. The area of crystal structure prediction is still in its infancy, so we need an alternative approach for practical applications in virtual cocrystal screening.
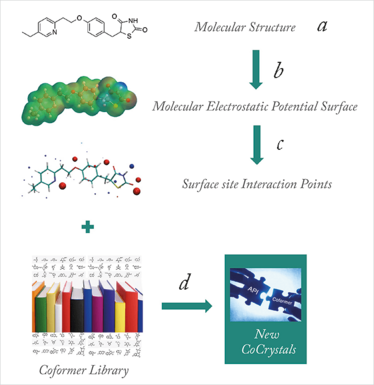
Figure 1. A virtual cocrystal screen. (a) the API is drawn in an extended conformation, (b) quantum chemistry is used to calculate 3D structure and MEPS, (c) the MEPS is converted into SSIPs, (d) the SSIPs are used to calculate the probability of cocrystal formation for each compound in a large library of potential coformers and the most promising candidates are selected for experimental testing.
A new approach
My research group and I (Chris Hunter) at the University of Cambridge have been working for over a decade on a project that uses a surface-based approach to non-covalent chemistry to describe the thermodynamics of intermolecular interactions in solution. The theory was first published in 2004 (8), and the approach has since been validated for H-bonds, halogen-bonds and aromatic interactions, for the effects of solvent and solvent mixtures on solution-phase molecular recognition, and for phase transfer properties like logP (9, 10, 11, 12). The great advantage of this approach is that it integrates theoretical ideas and experimental observations about intermolecular interactions in the gas phase, in the solid state, and in solution – all using a single unified framework.
Our approach assumes that the free energies of all intermolecular interactions in the condensed phase can be understood based on the gas phase electrostatic properties of the isolated molecules. Intermolecular interaction free energies are calculated using a set of discrete surface-site interaction points (SSIPs), which are characterized by parameters (α and β) that quantify the properties of positive (usually H-bond donor) and negative (usually H-bond acceptor) sites on the molecular surface. A condensed phase is treated as an ensemble of all possible interactions between collections of SSIPs, without considering three-dimensional structure, which tremendously simplifies the calculation of thermodynamic properties, such as the probability of cocrystal formation.
The application of this approach to cocrystal formation in the solid state follows the Etter principle that the structure of a crystalline solid is determined by a hierarchical organization of SSIP interactions. Accordingly, the strongest interaction is expected to be formed between the SSIP with the largest α (the best H-bond donor) and the SSIP with the largest β (the best H-bond acceptor), the next strongest interaction will be formed between the SSIP with the next highest α and the SSIP with the next highest β, and so on, until all of the SSIPs are used in the construction of the crystal structure. Summing the energies of these SSIP contacts gives the total non-covalent interaction energy of the solid. This can be done for the pure API, the pure coformer and for cocrystals of any desired stoichiometry, to establish whether the cocrystal is likely to be able to make an energetically more favorable set of non-covalent interactions than the components. This approach provides a very straightforward method for evaluating the probability of cocrystallization.
Moreover, it is not necessary to distinguish between different possible intermolecular interactions that are similar in energy. For example, if a molecule contains two very strong H-bond donors (D1 and D2) with similar α parameters and two very strong H-bond acceptors (A1 and A2) with similar β parameters, it does not matter whether the SSIPs pair one way, A1 with D1 and A2 with D2, or the other, A1 with D2 and A2 with D1. The overall energy of the crystal will be the same, even though the three-dimensional structures of the crystals will be completely different. This is the origin of polymorphism: different crystal structures with very similar energies. The fact that polymorphs generally differ by a few kJ mol-1 in energy demonstrates that structure does not matter as far as the energetics of SSIP contacts go.
The freedom to calculate energy without structure is the key to the speed of the SSIP approach and makes it viable to conduct virtual cocrystal screens on very large compound libraries. The method has now been experimentally validated for several different APIs and we believe it represents a powerful new tool in the armory of the solid-state formulation chemist (13, 14, 15).
- N. Blagden et al., “Crystal Engineering of Active Pharmaceutical Ingredients to Improve Solubility and Dissolution Rates”, Advanced Drug Delivery Reviews 59, 617–630 (2007).
- Food and Drug Administration, “Guidance for Industry: Regulatory Classification of Pharmaceutical Co-Crystals”, www.fda.gov (2013).
- European Medicine Agency, “Reflection Paper on the Use of Cocrystals and Other Solid State Forms of Active Substances in Medicinal Products”, www.ema.europa.eu (2014).
- E. Fischer, “Einfluss der Configuration auf die Wirkung der Enzyme”, Ber. Dtsch. Chem. Ges. 27, 2985 (1894).
- G. R. Desiraju, “Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis”, Angew. Chem. Int. Ed. 34, 2311 (1995).
- M. C. Etter, “Encoding and Decoding Hydrogen- Bond Patterns of Organic Compounds”, Acc. Chem. Res. 23 (4), 120–126 (1990).
- S. L. Price, “Predicting Crystal Structures of Organic Compounds”, Chem. Soc. Rev. 43, 2098–2111 (2014).
- C. A. Hunter, “Quantifying Intermolecular Interactions: Guidelines for the Molecular Recognition Toolbox”, Angew. Chem. Int. Ed. 43, 5310 (2004).
- C. S. Calero et al., “Footprinting Molecular Electrostatic Potential Surfaces for Calculation Of Solvation Energies”, Phys. Chem. Chem. Phys 15, 18262–18273 (2013).
- C. A. Hunter, “A Surface Site Interaction Model for the Properties of Liquids at Equilibrium”, Chem. Sci. 4, 1687−1700 (2013).
- S. L. Cockroft and C. A. Hunter, “Resolvation and Substituent Effects in Edge-To-Face Aromatic Interactions”, Chem. Commun. 3961- 3963 (2009).
- R. Cabot and C. A. Hunter, “Non-Covalent Interactions Between Iodo-Perfluorocarbons and Hydrogen Bond Acceptors”, Chem. Commun. 2005–2007 (2009).
- D. Musumeci et al.,“Virtual Cocrystal Screening”, Chem. Sci. 2, 883−890 (2011).
- T. Grecu et al., “Validation of a Computational Cocrystal Prediction Tool: Comparison of Virtual and Experimental Cocrystal Screening Results”, Cryst. Growth Des. 14, 165−171 (2014).
- T. Grecu et al., “Virtual Screening Identifies New Cocrystals of Nalidixic Acid”, Cryst. Growth Des., 14, 1749−1755 (2014).
“I was born in Dunedin in New Zealand, but then lived in Nigeria before my parents settled in Ireland. Following an undergraduate degree in Natural Sciences, I stayed at the University of Cambridge for a PhD in the Chemistry Department,” says Chris Hunter. He then decided to revisit New Zealand and took a lectureship in bioorganic chemistry at the University of Otago. He later moved to the University of Sheffield before returning to the University of Cambridge in 2014 as the Herchel Smith Professor of Organic Chemistry.
Born in Catalonia, Rafel Prohens grew up in Mallorca. “I received a PhD in Organic Chemistry from the University of the Balearic Islands and straightaway joined Chris Hunter's group at Sheffield University. In 2012, I founded CIRCE Crystal Engineering, a project to transfer Hunter’s approach on the study of intermolecular interactions into technological tools for the pharmaceutical industry.” Today, Rafel combines his roles as Scientific Director of CIRCE, Technical Manager at the University of Barcelona, and Research Visitor at the University of Cambridge.



















