
Finding Fingerprints of Biosimilars
After finally breaking into the US market, biosimilars have created a real buzz in the industry, but the best practice for demonstrating similarity can be daunting to say the least.
In most cases, it is relatively straightforward to prove that a generic small-molecule drug is the same as the originator product thanks to standard analytical chemistry and bioequivalence. Biologics, on the other hand, require head-to-head studies that prove the biosimilar is sufficiently similar to its originator in terms of structure, quality, safety and efficacy.
Biosimilars have been available in Europe for more than a decade, during which time the original 2005 biosimilars guidelines have been regularly updated – and in addition to overarching directives on quality, clinical and non-clinical requirements, there are product or class-specific guidelines for certain molecules (1). The European market is already being targeted by many non-European manufacturers, and numerous other countries worldwide have published and promoted their own pathways for biosimilars (many have chosen to adopt or adapt the European guidelines). The World Health Organization has also got in on the act by publishing guidelines on the evaluation of similar biotherapeutic products (SBPs) in 2009; supplementing these in March 2016 with a specific draft document on the evaluation of monoclonal antibody SBPs (2, 3).
The US was late to enter the biosimilars arena, with the introduction of the Biologics Price Competition and Innovation (BPCI) Act in 2010, which proposed the 351(k) pathway of the Public Health Services Act. It took another two years for the FDA to issue guidance for biosimilar manufacturers wanting to use this pathway, and further time for finalization; biosimilars have only started being approved in the country within the past year (4,5). As an accelerated pathway, 351(k) grants access to licensing based on a comparison with a reference product that has been approved via the standard 351(a) pathway. At the discretion of the FDA, a full suite of clinical trials may not be required for the biosimilar, as long as similarity to the originator is proven beyond “residual doubt.” Provision is made for a second tier – interchangeable biosimilars – if additional clinical studies are successfully conducted.
The Analytical Challenge
Celltrion cited a number of analytical methods in its successful European application for approval for Remsima (a biosimilar to infliximab). The primary structure was assessed using:
- liquid chromatography-mass spectrometry (LC-MS) peptide mapping
- LC-MS intact mass measurements
- amino-acid analysis/molar absorptivity studies
- N- and C-terminal sequencing.
The higher order structure was assessed using:
- FTIR
- differential scanning calorimetry
- circular dichroism
- free thiol and S–S studies
- antibody arrays
- X-ray crystallographic techniques.
The oligosaccharide profile, N-linked glycan, sialic acid and monosaccharide analyses were used to identify glycosylation patterns. Purity and impurities were investigated using:
- size exclusion
- chromatography (SEC)
- SEC with multi-angle light scattering (MALS)
- analytical ultracentrifugation
- capillary electrophoresis-SDS studies.
The charged isoforms were assessed using isoelectric focusing (IEC) and IEC-HPLC.
Proving similarity
Both clinical and non-clinical data are used to determine similarity. The basis of the biosimilar fingerprint is a statistical approach that demonstrates the two products are analytically similar, but some product attributes are more important than others. Data for the first tier, representing critical quality attributes, should include a statistical equivalence test to prove comparability, and the FDA recommends that these should include those attributes that pose the highest risk when different. A good example in some molecules may be the protein’s glycosylation pattern – the presence of sugars (oligosaccharides) attached to certain amino acid residues – or protein content. Second tier attributes are still important, but less critical, and quality ranges based on standard deviations may be appropriate for these. Those quality attributes in the third tier are the least critical, so graphical or raw data are likely to be sufficient.
The first step in proving biosimilarity is to determine detailed structural information for the originator molecule, which can then be used as a structural template for the putative biosimilar. It is important that many different batches of the originator are studied, as variation is likely to have occurred over time. The source of the reference product can also be an issue, particularly when developing a biosimilar for a global market, as some countries’ regulators will only permit proof of biosimilarity to a batch from another country if appropriate demonstration is made to show that it is indeed representative of the authorized product in the country of application.
Developing a fingerprint for a biosimilar involves the use of multiple orthogonal analytical techniques, with appropriate quantitative ranges.
The similarity toolkit
ICH Topic Q6B lays down test procedures for setting quality specifications for biological drug products. It demands multiple physicochemical and structural analyses, and is an excellent starting point when determining a strategy for proving biosimilarity. Six specification requirements for structural characterization are mentioned:
- amino acid sequence
- amino acid composition
- terminal amino acid sequences
- peptide map
- sulfhydryl group(s) and disulfide bridges
- carbohydrate structure (if appropriate).
There are also six specifications for physicochemical properties:
- molecular weight or size
- isoform pattern
- extinction coefficient
- electrophoretic pattern
- liquid chromatographic pattern
- spectroscopic profiles.
| Property To Be Determined | Available Methodologies |
| Amino acid sequence and modifications | Mass spectrometry, peptide mapping, chromatography |
| Glycosylation | Anion exchange, enzymatic digestion, peptide mapping, capillary electrophoresis, mass spectrometry |
| Folding | Mass spectrometry S-S bridge determination, calorimetry, hydrogen deuterium exchange and ion mobility mass spectrometry, nuclear magnetic resonance, circular dichroism, Fourier transform spectroscopy, fluorescence |
| PEGylation and isomerization | Chromatography, peptide mapping |
| Aggregation | Analytical ultracentrifugation, size-exclusion chromatography, asymmetric field flow fractionation, dynamic light scattering, microscopy, transmission electron microscopy |
| Proteolysis | Electrophoresis, chromatography, mass spectrometry |
| Impurities | Proteomics, immunoassays, metal and solvents analysis |
| Subunit interactions | Chromatography, ion mobility mass spectrometry |
| Heterogeneity of size, charge, hydrophobicity | Chromatography, gel and capillary electrophoresis, light scattering, ion mobility-mass spectrometry, capillary electrophoresis-mass spectrometry |
Table 1. Potential analytical tools.
Many different analytical techniques and tools can be used to obtain and collate this information, from classical chemical methods to newer, more advanced techniques, such as ion mobility mass spectrometry and hydrogen–deuterium exchange mass spectrometry (see Table 1 for a list). If the molecule is an antibody for instance, there are many types of interrogation that could and should be applied to structural comparison with the reference. The intact molecule can be studied, amino acid composition determined, and both N and C terminal sequencing carried out – a process that may require more than one peptide mapping enzyme digestion. The oligosaccharides attached to the heavy chain can be investigated, and the higher order structures determined.
Glycosylation is perhaps one of the most important post-translational modifications (PTMs) that occurs during the manufacture of a protein as it may affect the antibody’s efficacy and, in some cases, result in immunogenicity. PTMs such as glycosylation cannot be predicted from the gene sequence and have to be determined experimentally. Furthermore, the unpredictable addition of sugars greatly adds to the heterogeneity of the biologic medicine. As an example, just one immunoglobulin G type molecule has been estimated to have 3x108 potential variations (see Figure 1). One technique that can be applied here is electrospray ionization (ESI) mass spectrometry, which can provide insight into the number and nature of carbohydrates that are attached on both the reference drug and the biosimilar.
ICH Q6B describes the need to study the carbohydrate content, the structure of the carbohydrate chains, and the glycosylation sites. Strategies analogous to those used for peptide mapping can be applied; for example, the glycoprotein can be analyzed intact or digested to form glycopeptides to detail the sites of glycosylation. Carbohydrate can also be released from the protein backbone. The resulting glycans can then be analyzed using chromatography and mass spectrometry.
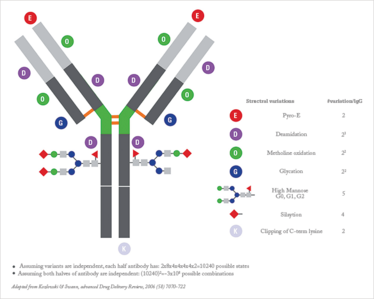
Figure 1. Example of complexity in the case of an antibody.
Higher order structure
The conformation of the biologic also has a bearing on its activity and is another important area of investigation when developing a fingerprint for biosimilarity. Again, many techniques – both qualitative and quantitative – can be applied to determine higher order structure. One of the most commonly applied quantitative techniques is circular dichroism, which is sensitive to helix content, providing information about both secondary and some tertiary structure. On the down side, the presence of buffers in the formulation can interfere with the results. Fourier transform infrared spectroscopy (FTIR) is another quantitative method for secondary structure determination that is sensitive to sheet content and less likely to be affected by buffers.
Both intrinsic and extrinsic fluorescence techniques are used – the former for local tertiary structure, and the latter for surface hydrophobicity – but only give qualitative results. Other qualitative methods include differential scanning calorimetry, which looks at thermal stability, and UV-vis spectroscopy for local tertiary structure. An emerging technique from research applications, hydrogen–deuterium exchange mass spectrometry, highlights details of dynamics, conformation and interactions, but is expensive and has significant data processing requirements. Another technique more normally applied in a research setting is two-dimensional protein nuclear magnetic resonance.
The way that biologics oligomerize and aggregate must also be studied. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) is an inexpensive but low-throughput tool for assessing aggregates, and dynamic light scattering (DLS) can be used to look for high-molecular weight aggregates. Oligomers and aggregates can both be investigated using sedimentation velocity analytical ultracentrifugation (SV-AUC) and size-exclusion chromatography with multi-angle light scattering (SEC-MALS), both of which give
quantitative results.
The science of safety
It is not sufficient merely to assess comparative structure: comparative functional assays also have to be performed. Suitable quantitative biological assays have to be developed and run to link product attributes with biological properties – and the results for the originator and biosimilar must correlate well if similarity is to be accepted by regulators. The assay must also be able to assess properties appropriate to the nature of the biosimilar. Again, a range of techniques can be applied, including biochemical assays, such as ligand binding, immunoassays, enzymatic assays and radioimmunoassay studies. Others are cell-culture based, including cytotoxicity, cell uptake, proliferation, secondary messenger and PCR-based functional assays.
The chosen techniques, both structural and functional, will vary from one biosimilar to another. However, the resulting information should always cover a sufficiently wide range of parameters to give regulators confidence that the biosimilar will behave in a similar fashion to its reference product in patients. For an example of how many different techniques may be needed for one product, see “The Analytical Challenge”.
With the inevitable variability between biologic products manufactured in different cell lines, careful comparative studies are essential if regulators are to be convinced that a biosimilar is both safe and effective. By applying multiple orthogonal analytical techniques to both the reference originator product and the biosimilar, including functional studies, an all-important fingerprint of biosimilarity can give confidence that patients will not be adversely affected if they are prescribed a biosimilar instead of the originator product.
- European Medicines Agency, “Multidisciplinary: biosimilar”. Available at: bit.ly/1trteeH. Accessed June 13, 2016.
- World Health Organization, “Similar biotherapeutic products” (2014). Available at: bit.ly/1XiRR8W. Accessed June 13, 2016.
- World Health Organization, “Guidelines on evaluation of monoclonal antibodies as similar biotherapeutic products (SBPs)”, (2016). Available at: bit.ly/1ULeTlr. Accessed June 13, 2016.
- FDA, “Information for Industry (Biosimilars)”, (2016). Available at: 1.usa.gov/1wAiQzJ. Accessed June 13, 2016.
- S Sutton, “The big break for biosimilars?”, The Medicine Maker, 6, 18-25 (2015). Available at: bit.ly/25X2397.
Fiona Greer is Life Sciences Global Director, Biopharma Services Development at SGS.



















