Formulation Best Practice
Are you in need of know-how about formulation best practices? In this technical roundtable, industry gurus Frank Romanski, Derek Bush and Kishor Wasan share their views and tips for success.

Frank Romanski is Global Technical Marketing – Pharma Solutions, at BASF, Germany.

Kishor Wasan is Dean of the College of Pharmacy and Nutrition, University of Saskatchewan, Canada.

Derek Bush is Manager, Product Development, at Catalent Pharma Solutions, USA.
How should we approach the formulation of a poorly bioavailable API?
Wasan Kishor: It is important to remember that there is no magic bullet – no one excipient that can fix all bioavailability problems. You need to take a case-by-case approach and follow an iterative process. First, define the precise problem you are dealing with – is it a solubility issue, or a gut metabolism issue, or a release kinetics issue? Then assess the physical chemical properties of the API in question – are they compatible with an LBDDS? If so, you can proceed to the next step: identifying lipids that could both overcome the identified barrier to drug efficacy and be compatible with your API.
Derek Bush: First, establish whether the bioavailability issue is related primarily to solubility or permeability – or both. If you don’t have those data, you need to conduct in-silico and in-vitro screening tests to establish if the API is Developability Classification System (DCS) Class II or Class IV. For Class II compounds, the next step is to identify the key factor that limits solubility – i.e., Class IIa (dissolution rate limited absorption), or Class IIb (solubility limited absorption). Addressing a Class IIa issue is relatively straightforward; micronization, milling or nano-milling technologies may help, as may lipid-based formulation approaches. Options for Class IIb compounds are more complex, and while it may be possible to improve solubility through covalent modification of the API, creating salts, or isolating polymorphs, the classic route for these high logP compounds remains lipid-based formulation. DCS Class IV, however, is the most challenging category; in addition to all the Class IIb hurdles, you have a permeability problem – the basis of which is often much more significant than simply first order absorption kinetics. Causes of low permeability include gut metabolism, P-glycoprotein (P-gp) efflux, and protein-API interactions in the gut. Significant experimentation may be required to clearly define the basis of low bioavailability, but a clear understanding of the problem you face is essential to guide formulation decisions. Excipient choice may vary according to whether you need to stabilize the API, or stimulate lymphatic transport, or inhibit PGP, prevent drug metabolism in the gut, or control the drug release profile so as to protect the API from gastric acid. You won’t know which route to take until you understand the permeability constraints. That said, remember that the required dose will have a significant impact on your strategy, and that this may change during the development process – a Class IIb molecule can easily be shifted into Class IIa, or even Class 1, by reducing the target dose. For example, as you improve API permeability you may find that the required dose is reduced. Consequently, the formulation may become more of a Class I than a Class IIb issue.
Frank Romanski: Most pharmaceutical companies want their drugs formulated as an oral dosage form, either a tablet or capsule, and they want the formulation available to achieve good oral bioavailability (>90 percent). For low bioavailability compounds, the most successful commercial approaches include preparation of a solid amorphous dispersion through hot melt extrusion or spray drying (techniques in which API is dissolved in a polymer matrix prior to forming a solid tablet), and lipid-based formulations primarily encapsulated in softgels. These newer amorphous dispersion techniques can be highly effective for the right APIs. For example, many years ago, as a student, I was working on the industry standard model poorly water-soluble drugs, fenofibrate and griseofulvin. We developed complicated nanosuspension formulations comprising countless different ingredients with multi-stage production schemes. When I moved to BASF, it took only a fortnight to adequately solubilize those same drugs – and at ten-fold higher concentrations than we had previously been able to achieve! Formerly novel techniques, such as solid amorphous dispersions and complex lipid systems (e.g. self-emulsifying drug delivery systems – SEDDS and SMEDDS) are becoming more and more mainstream solutions, and work well with existing oral dose technologies. Yet, the challenge remains that these complex systems rely heavily on functional excipients, and these difficult to understand API-excipient and excipient-excipient interactions are what drive these new techniques to higher levels of success than previously attainable.
How do you choose from the range of excipients that might be suitable for a given API?
KW: Once you have defined the barrier for your drug in terms of its efficacy/toxicity ratio, you can start thinking about the most appropriate excipients. If the API is crystallizing out in the GI tract then a lipid formulation may provide an answer. Similarly, incorporating API into a lipid may help protect acid-labile drugs until they reach the more benign environment of the small intestine. So, first understand the problem you are facing, then assess the physicochemical properties of your drug – and only then start making decisions about excipients.
DB: Unless you are pursuing a suspension formulation approach (largely limited to compounds that are at least moderately soluble in biological media), the first goal is almost always to dissolve your drug and keep it dissolved. Therefore, you should undertake a solubility screen with broad classes of excipients to identify those that give maximum API solubility. If the outcome of that study is that you can only achieve very poor solubility levels relative to the required dose, you may have to consider API modification, or assess the potential of suspension formulation. The latter, however, is not suitable for compounds of low aqueous solubility and low permeability, especially where the dose requirement is high. Once you have an API of sufficient solubility, you may need a second tier solubility screen to assess excipient compatibility. In this step, you take the subset of excipients that gave the best solubility in the first screen, and test API stability with these excipient candidates under accelerated conditions. These two solubility screening studies are key in determining your formulation pathway.
FR: From an excipient supplier perspective, quality is very important, including assurance that the material was produced in accordance with appropriate pharmaceutical monographs, such as the USP, Ph. Eur. and JP/JPE, and produced under IPEC GMP quality standards. Apart from anything else, this allows for proper change control process, including identification and communication of raw material changes, which could impact product quality. This is especially important for lipids because of their broad specifications – changes in the lipid raw material supply chain and/or subsequent synthesis, purification and stability can impact the final characteristics of the product, yet still be within comparatively broad product specifications. It is important to ensure that an excipient supplier is committed to the quality standards of the pharmaceutical industry, as drug manufacturers need critical information – and as early as possible.
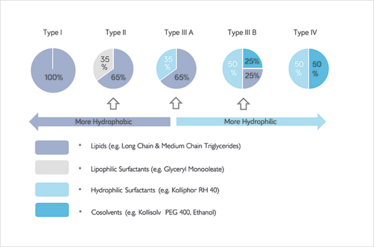
Figure 1: The lipid formulation calssification system. Complex multi-phase formulations are typically in Type II and III A/B. © BASF. Pouton et al., LFCS Consortium
What is it about lipids that makes them a compelling formulation option?
KW: Lipid excipients are special in that the body does not see them as foreign products. Other excipients may trigger non-specific effects, but triglycerides and their breakdown products are part of the normal physiological environment and, hence, not associated with allergic reactions or other effects – one less thing that you have to worry about! Another differentiator is the amount of options they give you – the lipid category is very broad, so you have many variants to choose from, and many different possibilities in terms of molecular weight, melting point, and so on.
DB: The great advantage of LBDDS is that, being a natural dietary component, they are trafficked by natural systems. Thus, in a LBDDS, drugs are piggybacked across the GI tract in a natural metabolic process. In this way, they not only enhance absorption and permeation, but also provide an excellent safety profile.
FR: Lipids have distinct advantages in their applicability to hydrophobic, high logP molecules. They also demand uniquely sophisticated expertise. For example, lipid formulations tend to be non-equilibrium systems; accordingly, changes in ambient moisture or temperature may affect formulation thermodynamics and ultimately lead to stability problems. This specific challenge with lipid formulations makes it very difficult to predict their behavior over time, so you need significant experience to manage this issue.
Are the advantages of lipids well-understood, or do misconceptions remain in the industry?
KW: The industry has a better appreciation of LBDDS than they once did, but they still have a way to go. The lipid-based focus group at AAPS, which started about 20 years ago, has helped to educate the pharmaceutical community on the use of lipids. Furthermore, many papers are now available for the naive formulator who is considering lipids as a potential option. We ourselves recently published a paper showing examples of lipid-formulated FDA-approved products (1).
DB: People are aware of the advantages of LBDDS in terms of increasing the bioavailability of compounds with poor aqueous solubility. Less broadly appreciated are the complexities of lipid formulation – it’s not as straightforward as dissolving API in triglyceride and surrounding it with a softgel capsule. In particular, lipid raw materials are mixtures of multiple components and can be very variable in the ratios of the components depending on the source. We’ve actually seen cases of the exact same medium chain triglyceride, sourced from two different suppliers, giving different invivo clinical results depending on its manufacturer. It turned out that the fatty acid composition and breakdown products of these two products were different, even though they were supposed to be the same medium chain triglyceride – and met the corresponding USP requirements for being defined as such. More generally, in a given medium train chain triglyceride, the ratios of different fatty acids (for example, C6, C8, C10 and C12) can differ substantially; for instance, C6 and C12 vary by 20 to 30 percent from one supplier to another. Because of this, formulators need to emphasize the quality by design (QbD) approach – what are your critical material attributes, and how do they influence your critical quality attributes? Understanding the variability of your formulation materials, therefore, is a key part of lipid-based drug development. It not only affects aspects such as stability at certain processing temperatures, but also can influence product behavior in the human body, e.g., in terms of susceptibility to digestion. Another point that may be insufficiently recognized is that lipid excipients are dynamic – they change as they progress through your body, and without careful handling they will also change during the manufacturing process. Oxygen exposure, light exposure, and heat exposure can all affect lipid excipients in some way.
FR: People do not always appreciate the sophistication of lipid formulations. As lipidic formulations have evolved to multicomponent, complex systems, formulators rely on the same “magic bullet” excipients, such as a single surfactant as the only excipient. Unfortunately, this is a fundamentally ineffective approach. For example, in more complex formulations such as self-emulsifying systems (e.g. SEDDS, SMEDDS), a formulator may now need multiple ingredients with specific functionality, including, but not limited to: primary emulsifier, secondary emulsifier, crystallization inhibitor, co-solvent, and so forth.
One particular area which needs significant improvement is the basic concept of HLB, also known as the hydrophilic-lipophilic balance. The idea, which has been around since 1949, is that the HLB number assigned to a given lipid system suggests that a single surfactant with this magic HLB value should ideally stabilize the system. However, in practice, while surfactants may have similar HLB values, they may also have vastly different chemistries, conformations, geometries, and capacities to interact with other molecules; and so, the idealized magic number is of little value. Strict reliance on the HLB number is perhaps one of the common misconceptions I see frequently with formulators of lipid formulations, regardless of application. Consequently, my advice is this: understand that the HLB value is a starting point only, and that ultimately other characteristics, such as excipient structures, chemistries and interactions, must be considered.
What advice would you give to those embarking on a lipid formulation project?
KW: A common mistake is failing to do the necessary homework. I would strongly advise formulators to assess the physicochemical properties of the API to confirm the applicability of the lipid-based formulation route. Failing to appreciate that not all compounds are appropriate for lipid excipients – and expecting the addition of a lipid to be like waving a magic wand – is a common error. It is also unnecessary because the key criteria for particular formulation objectives are reasonably well-known. For example, if firstpass metabolism is a problem, you may want to improve drug bioavailability by routing drug uptake via lymphatic transport, such that the drug is absorbed via the mesenteric lymph duct rather than the liver portal vein. If so, you should check your compound against known criteria for lymphatic uptake: does your API have a molecular weight of less than 500? Does it have a logP greater then 5? Does it have a triglyceride solubility value of 50 mg/ml or more? And does it partition into very low density lipoproteins? If so, you have a good chance of improving bioavailability via lymphatic transport, but not every drug candidate fits this profile. So make sure you understand the constraints of your system before making formulation decisions.
DB: Invest in upfront investigation to fully understand your excipients. If necessary, carry out tests to assess the effects of oxygen, light and temperature on the excipient itself, not just on the API; in many cases, product instability is a consequence of degradation of lipid excipients rather than due to true API instability. Fortunately, instability of lipid excipients can usually be addressed by the addition of an antioxidant. In fact, API stabilization is a secondary consequence of antioxidant addition, as the primary function of antioxidants is to stabilize excipients under the specific processing conditions required to solubilize and deliver the API. So those working with lipid-based excipients should focus on fully understanding them, with particular regard to their stability requirements – it’s a key formulation point. FR: Decide as early as possible what kind of solubility or bioavailability problem you are facing and what your formulation options are for dealing with it, and then choose the right partner to work with so as to devise the simplest possible formulation system. Above all, remember that there’s no “one size fits all” approach.
What challenges and mistakes are commonly seen when making lipid-based formulations?
DB: People sometimes forget about the need to understand how lipid-based excipients behave after dispersion in intestinal fluid. What happens to your formulation in an aqueous environment in the presence of pancreatic enzymes – how is it digested? You need to know what kind of changes in API solubility you would expect in that environment. The solubility may not change, but equally it could go up or down (and in some cases, it can go down quite dramatically). Studies on solubilization kinetics during and after lipid digestion are very important, but are easily missed in the development process. It’s not a difficult step, but sometimes it is overlooked.
FR: : A big challenge that is often under-appreciated relates to the dynamic nature of LBDDS. As noted earlier, these systems, particularly when encapsulated, are subject also to environmental fluxuations. In a softgel form, for example, water may pass between the gelatin capsule shell and the formulation it encloses (such movement is driven also by temperature changes). Oxygen and other components (e.g. plasticizer) may also move similarly. These fluxes may shift the thermodynamics of the system and thus must carefully be taken into account in order to ensure long term stability of a lipid formulation. Predicting how these formulations will behave over the long term is therefore very challenging. And again, the most common error of all is to assume that the HLB number will magically identify a surfactant that solves all your formulation problems. The HLB value cannot apply equally to all surfactants – it’s no more than a rule of thumb.
When should companies start planning the final dosage form?
DB: The earlier they start to think about permeability and solubility, the better! I’ve noticed a consistent bias among API manufacturers – they always seek to maximize the aqueous solubility of the drug. As a consequence, they usually synthesize a very specific salt form that is soluble in an aqueous environment, but not in lipids. So, should you later find that you need a lipid-based formulation to enhance solubility or bioavailability, you have a problem, because it’s very difficult to formulate a lipophilic form of that hydrophilic salt. If you instead formulate the API as a free base (assuming it has an amine group) at the start of your development process then it is far easier to make a lipid-based formulation. This is why I usually advise our customers to make the free base form of the compound, rather than the salt form.
FR: You should always make strategically important decisions as soon as you can. Whether you go for a lipid formulation, a spraydried formulation, or a hot melt extrusion formulation, you need fast decisions if you want to be fast to market. And it is possible to make these decisions quickly – the characteristics of the API are known very early in the process, and will indicate the most appropriate dosage form – hence, guiding decisions on excipients that will favorably interact with the API to allow maximum stability, solubility and bioavailability.
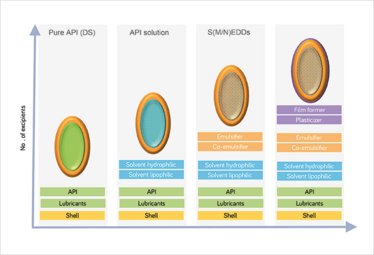
Figure 2: Softgel excipients. As complexity of LBDDS increases, so too does the excipient need. © BASF
Any final advice?
KW: Take the time to do the background work to fully understand your drug and the nature of the problem you need to overcome. You won’t regret it.
DB: Begin with the end in mind. Clearly defined goals and objectives will help you to produce a development plan, which is very important. A key part of the exercise is to understand from a regulatory standpoint where you are going to end up, even if that is 10 years away. It can be disastrous to get 80 percent of the way through a development program and then realize you have to repeat some work or do an additional trial; it’s always better to spend more time up front. From the very beginning, you should be matching critical attributes with the critical goals that you need to achieve. This may require you to seek expert advice at an early stage so that you understand the regulatory environment and the type of filing strategy you should undertake for your particular compound. In brief: know where you want to end up and work backwards from there!
FR: Don’t ignore excipient suppliers. We have significant knowhow regarding lipid formulation, manufacture and stability, and can guide companies through the many issues arising from the broad specifications associated with lipid and other solution enabling ingredients.
Derek Bush: Development of in-vitro screening methodology to guide clinical studies
A recent project required us at Catalent to generate in-vitro in-vivo correlation values for a Phase 1 clinical-stage molecule with high logP and low aqueous solubility. We started with a classical dissolution approach, in which we put the dosage form into an aqueous medium and analyzed samples at 15 minute time points. The data showed a linear increase over time, and on this basis we made decisions about the formulation for the in-vivo work.The subsequent animal pharmacokinetics data, however, showed hardly any correlation at all with our invitro results clearly, we had a problem. We began testing alternative methodologies, in particular fibre optic dissolution testing. This technique allows very frequent measurements of API concentration (every 2 or 3 seconds, if necessary) and it revealed a huge supersaturation parachute effect occurring in the first 15 minutes. During this time, the concentration rapidly spiked to a level much higher than the theoretical equilibrium solubility. It stayed high over most of this period, but came down by the time we would take our first sample in the classical dissolution testing approach – which is why we’d been missing the big spike. For a DCS Class 2 compound, which is purely solubility-limited, not permeability-limited, this was a very important finding. In fact, it fundamentally changed the way we designed the formulation and excipients for that API. This study was a key learning point for us, and made us rethink our invitro screening methodologies to ensure that we always made the best decisions possible for the molecule and for the product.
Kishor Wasan: Collaborative venture involving manufacturer, biotech and academic group
We have been working with iCo Therapeutics and Gattefosse on an oral formulation of amphotericin B (an anti-fungal agent): Gattefosse provides the lipids, we provide lipid expertise, and ICO Therapeutics undertakes formulation and Phase 1 clinical development. This is a fantastic example of a collaboration between an academic group, an excipient supplier and a biotech company, all contributing different resources and skills, and all working together towards a common goal. So far, preclinical studies confirm the ability to deliver amphotericin B orally and in high enough tissue concentrations to elicit biological activity without significant toxicity. iCo is due to start dosing human subjects in a Phase I study in Q4 2017.
Frank Romanski: Phase diagram for microemulsion design
We have done a lot of case study work in the field of microemulsions. These are specific, uniquely rich systems where surfactant and cosurfactant are in equilibrium with both water and oil – not a true emulsion of oil droplets in a continuous water phase, but a bicontinuous phase of oil and water tied together by tremendous amounts of surfactants. Microemulsions are unusual in a number of ways; their droplets are actually smaller than those in nano-emulsions, and unlike normal emulsions, which are cloudy or milky, they are as clear as water. But their critical advantage is that – unlike normal emulsions, which will eventually separate into oil and water phases (formulation only delays this outcome) – microemulsions remain emulsified permanently. The advantages for drug formulation are obvious. Furthermore, not only are microemulsions very stable inside the capsule, but they can be designed to form other structures, such as nano-emulsions, after capsule disintegration. This gives formulators the option of ensuring the API is presented to the gut in a form designed for maximal bioavailability. The only disadvantage of microemulsions is that they are very challenging to make – you need exactly the right mix of surfactants if you are to produce a perfectly stable system, and it is very difficult to know which oils to pick. For that reason, we undertook a tremendous amount of highthroughput chemistry, assessing different pairings of surfactants and oil phases, from which we mapped out an entire phase diagram. This allows us to quickly and efficiently identify components that will generate thermodynamically stable microemulsions. From that starting point, we can tweak the system to achieve the required characteristics; for example in terms of drug release profiles.
- R Savla et al., “Review and analysis of FDA-approved drugs using lipid-based formulations”, Drug Dev Ind Pharm, 6, 1-16 (2017). PMID: 28673096



















