
How Transparent Are Trials?
Only 50 percent of clinical trial results in Europe are reported, but big pharma shares far less than half the blame
UK members of parliament recently expressed concerns that only around half of UK clinical trial results are being reported (1). And according to EU Trials Tracker, results for only 51.9 percent of those trials due in Europe have so far been reported (4072 out of 7846 trials) (2).
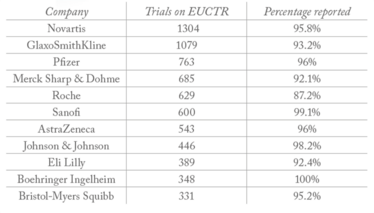
Table 1. Big pharma sponsors with the largest number of trials on EUCTR.
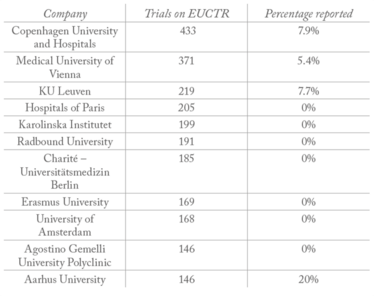
Table 2. University/hospital/research institute sponsors with the largest number of trials on EUCTR.
All clinical trials on the European Union clinical trials register (EUCTR) must report their results within a year of completion. And though very few companies report 100 percent of their trial results (meaning there is work to be done, see Table 1), big pharma is far from being the worst offender; hospitals and universities are significantly less likely to report their results – with many reporting no results at all (see Table 2).
- BBC News, “Unpublished medical research ‘a threat to public health’” (2018). Available at bbc.in/2Ror8I6. Accessed November 8, 2018.
- EU Trials Tracker. Available at eu.trialstracker.net. Accessed November 8, 2018.

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















