
Language as Quality Control...
... or sonicate until the cockroaches disappear. When writing is central to the job of assuring health, safety, and quality, can you afford to be loose with language prone to misunderstanding? Why take a chance? Here are a few strategies to edit your written work for quality control.
Advice on writing is plentiful – whether for writing short stories or scientific articles: “Avoid vague statements, jargon and laboratory slang” (1); scientific writing must be “precise and unambiguous”(2); “the writer should so write that his reader not only may, but must, understand” (3); and “read closely and revise your own manuscript at least three times before submission” (4).
But where do you start when editing your own or someone else’s work? What do you look for? In “How to Fail an FDA Quality Audit”, Mort Levin states that based on “sampling statistics, if 1 percent of [written] procedures have problems, the probability of finding one or more in a group of 100 is 63 percent” (5). In other words, if you find one cockroach, dozens more surely lurk in the dark. So, my advice for a first editing step is to examine the language and look for the cockroaches; they thrive in vague wording, jargon, and ambiguity.
Non-specific language
When introducing a unit on language in writing seminars, I often start with an excerpt from a pharmaceutical standard operating procedure I once edited: “Inspect the vials periodically for microbial growth.” Is the directive “periodically” up to quality standards? Many defend it. Since this vague word is found in so much documentation, some believe there must be a successful strategy behind it. I have heard explanations, such as “you don’t want to be too clear because if the FDA comes in and you’re inspecting the vials on the eighth day when it should be done on the seventh, you’re in trouble”. Would the FDA really be fooled? Use “periodic” and you may very well have sent the invitation for a visit. So, how to edit this? Ask the chemist or microbiologist to explain the purpose of inspecting the vials and the best time to make the inspection to assure the intended outcome. Revision: Inspect the vials for microbial growth every seven days after incubation.
This is not an isolated example of unclear scientific writing. Examine every batch of writing intended to provide specific direction and quality assurance, and you will find more:
- “All exposed jewelry must be removed if in close proximity of operating equipment.” How close is close proximity? (And close proximity itself is redundant.)
- “Select 10 biohazard autoclavable hazardous material bags (or equivalent).” Would someone new to this method know what’s autoclavable, as well as its equivalent?
- “Objective: To detect any obvious high and low filled vials.” What’s obvious? Could the high and low range be measured?
- “Some solvents can damage the appearance and function of the instrument.” Which solvents? How will the reader know?
- “Unless otherwise directed, non-company personnel are required to wear safety glasses.” Directed by whom or what? And who are non-company personnel?
- “This uncalibrated capper count appears to be more accurate than the average tray count.” Is the uncalibrated count more accurate or not? What’s the basis of this appearance?
- “When necessary, identify isolates using an appropriate microbial identification system.” When would this be necessary, and what system would be appropriate?
- “Minimize reaching over open containers, syringe baskets, etc. on the tables and the number of people moving at one time in the rooms during operations in the class 100 areas.” Is reaching over sterile components acceptable at all? How many times and how many people moving at one time jeopardize product quality? What other types of items could etc. refer to?
Not all of these examples will jeopardize quality or safety or prevent a scientist from reproducing another’s results. But why take a chance?
When editing for clarity, ask yourself, is this specific enough, will this produce consistent results, could two people interpret it in more than one way? If it’s fine as is, move on. If there is a probability for misunderstanding, then rewrite so readers must versus may understand. Vague language shifts the burden or responsibility to the reader, who will either find out what’s necessary, appropriate, obvious, or guess and make a decision based on subjective, loosely chosen language.
Jargon – the bottom line, if you will, of synergistic scenarios
Jargon is so pervasive in English that it’s often hard to recognize or see what’s wrong with it and why writing guides decry it. We use jargon, as we do slang, in everyday conversation, not realizing it – I’m going to “scoot to” the store, “pick up” groceries, and “whip up” something for dinner. In science, the jargon might sound more technical, but it’s just as potentially vague, imprecise, and troublesome. Here are some examples:
- “The new study has been performed for optimization purposes.” Sounds great; who isn’t for optimization? The study’s objective, however, buried pages later in the study, was to increase a product’s shelf life by six months.
- “Products in question will be formulated and held at their required storage parameters per their respective master records by the Formulation Department.” Who doesn’t love a parameter... and its melodious sound on the tongue, sprinkled with a little “per” and “respective”? But the writer could have stated the point directly and simply – the products will be formulated and stored as their master records require.
The biggest cockroach in the jargon family is “utilize” and its fellow hatchlings “utilizing” and “utilization”:
- “Laboratory documentation was reviewed to determine the number of samples that were utilized in the testing performed.” Possible revision: We reviewed the documentation to determine the number of samples tested.
- “Biologically, perchlorate interferes with the utilization of iodine and disrupts hormone production by the thyroid gland.” What’s the actual causation described here? What is utilizing the iodine and the relation between iodine and the thyroid? When writers make a commitment to avoiding the jargon (in this case, not using utilization), they choose more descriptive verbs and concise phrasing: Perchlorate interferes with (or inhibits) the thyroid’s uptake of iodine, which is essential for hormone production.
Again, because jargon and imprecise language are so pervasive, it would seem the scientific professions condone it, even though all scientific style guides – whether from the American Chemistry Society or the Council of Biology Editors – devote chapters that discourage it. Why, when so well documented in these sources, is jargon hard to exterminate? Possibly because it sounds (to the writer) intelligent, sophisticated, in the know, and part of the profession. Using jargon may make the writer feel good. It’s also easy to use.
Nonetheless, jargon makes the job of reading more complicated, abstract, and longer than necessary. Is there a compromise? For an editor, I’d say no. Scientific peer reviewers, however, may be more tolerant, focusing on accuracy, precision, or reducing the probability for failure. If the jargon creates inflated, sluggish prose that is difficult to read, fine (but still try to improve it). But if the language is potentially ambiguous, not technical and accurate enough (as illustrated in this article’s examples), simpler, more specific language is a must.
It’s always been done this way... and they should know this
The most difficult obstacle to overcome as a writer and editor might be a resistance to change based on an unfounded belief that because a practice is so common, it must have been proven scientifically infallible. When it comes to writing, it can be difficult to prove a case for applying best practices because the metrics are often subjective. Even though an editor can demonstrate jargon’s shortcomings or how to delete wordiness, it’s difficult to overcome statements like, “that’s just our style, this is the standard language we always use, and if they are working in this field, they should know this.”
Two common examples I’ve found yield some metrics and support for avoiding jargon and using plain yet specific language. One is based on research; the other, on interviews with QA personnel in R&D and manufacturing and their on-the-job experiences.
- “For external use only.” A study of prescription drug warnings at Northwestern University (Evanston, Illinois, US) has centered on how to improve the warnings – the standard warnings now in use – to assure patient comprehension and reduce the risk of errors (6).
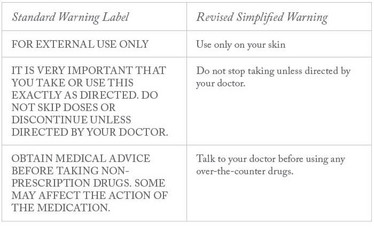
Table 1. Standard prescription drug warning labels alongside drug warnings that were rewritten in more plain language with less text
The research compared adult patients’ responses to current standard drug warning labels with responses to drug warnings that were rewritten in more plain language with less text. See Table 1 for three of the nine labels and revisions that the study used. The researchers found an 80.3 percent rate of correct interpretation of the standard drug warnings and a 90.6 percent correct interpretation of the revised simplified ones.
- “Sonicate until dissolved.” The industry need for simple, direct, and unambiguous instructions is no less important for reducing the probability of misunderstanding in methods, lab protocols, or analytical studies. The threat of lab jargon – assuming stock phrases and language are as universally understood as, say, the symbol for benzene – is also no less intense.
In reviewing a method, ask a chemist, does “sonicate until dissolved” need to be explained? You might hear a sigh or a statement like “why should we have to tell an analyst that?” For a standard operating procedure (SOP), this language leaves the step open to interpretation and one that can be completed differently each time by the same person; one analyst will use the sonicator to prepare a sample for 15 minutes over a coffee break, but another may take an hour for lunch...
QA managers and directors attest that the latitude embedded in “sonicate until dissolved” is prone to error. It can be cited as the cause for low assay results and results out of specification, even though nothing was wrong with the product. Such variances waste resources, time and money (through additional testing), delay the release of product or, in stability testing, delay reporting the results to the FDA. Why take the chance?
There’s no perfect solution, but there are ways to reduce this risk – through language and science, and by making instructions method-specific, not analyst-dependent. A possible translation in plain language might be “prepare the solution using x and z. Sonicate it until the solution is clear and no particles can be observed.” However, for photosensitive testing that uses amber glassware, an analyst can’t see what’s dissolved. A more reliable and consistent approach would be to base the directive on experiments that identify the time required for this method to eliminate possible variances in results, and state it in unambiguous language: When using Method A, sonicate the solution for 15 minutes.
There is no such thing as a good writer
To allude to a line in the sales profession, writers are only as good as their last written communication. Writing is work, and no simple formula is going to produce the quality product desired every time. Rigorous editing and rewriting will increase the chances. So give yourself a hard time – and ask others who review your work to do the same. As the legal scholar Bryan Garner advises, “Review your writing ungenerously, as a harsh critic might... If you approach your own writing mercilessly, your readers are sure to be merciful” (7). Great advice, too, for professionals involved in the health and safety of the public. Why take a chance?
Steven Schultz is President of Writing at Work (email: [email protected]), Oldsmar, Florida, USA.
This article was originally published in The Analytical Scientist (www.theanalyticalscientist.com), a sister publication to The Medicine Maker.
- M O’Connor, “The scientist as editor: guidelines for editors of books and journal”, Wiley, New York, USA (1979).
- “The ACS style guide: effective communication of scientific information”, American Chemical Society, Washington, DC, USA (2006).
- A Bierce, “Write it right: a little blacklist of literary faults”, Bowman, New York, USA (1934).
- S Lehotay, “Manuscript Master Class,” The Analytical Scientist, 35, 46–49 (2015).
- M Levin, “How to Fail an FDA Quality Audit”, Levin, Inc., Natick, Massachusetts, USA (1996).
- MS Wolf et al., “Improving prescription drug warnings to promote patient comprehension,” Arch Intern Med, 170(1), 50–56 (2010). PMID: 20065199.
- 7. BA Garner, “The elements of legal style”, Oxford University Press, UK (1991).
Having published articles on the 14th century sculptor Claus Sluter and the 20th century poet HD (Hilda Doolittle) 25 years ago, Steve has since enjoyed working on science and engineering projects as principal consultant at Writing for Work – whether editing ion chromatography methods or describing the uses of spectrophotometers for pharmaceutical and life sciences labs. Steve says, “Studying a poem and editing a scientific article is the same work. You’re intensely analyzing words to figure out what something means as well as what it doesn’t. You’re also using language to inform others about something they didn’t know, had not experienced, or couldn’t see before. It’s also exciting to know that this work often brings about specific changes for the better – and that someone is actually going to read it.”
Steve also enjoys the different teaching dynamics of running writing seminars for scientists and other technical professionals, as part of company training initiatives or at industry conferences such as Pittcon, which has featured his technical writing short course for the past nine years. He looks forward to Pittcon 2017 that will be held in his hometown Chicago—a city rich and proud of its world-renowned contributions to architecture, science, literature, and its unabashed use of direct, colorful language in taking care of everyday business.



















