Let's Go In Vitro: Scalable 3D Models in AI Drug Discovery
AI is generating new drug discovery solutions for a rapidly changing industry
Jan Lichtenberg | | 3 min read | Opinion

I’ve always been passionate about the benefits of scalable 3D in vitro models. With AI continuing to be incorporated into drug discovery, I want to highlight how these models are indispensable building blocks. These advanced models are not just enhancing the predictive accuracy of preclinical studies; they are also driving the successful implementation of AI algorithms in drug discovery processes because they deliver the breadth and depth of data needed for machine learning.
The success of AI in drug discovery hinges on the availability of high-quality, biologically relevant data. AI algorithms require broad and deep datasets to learn and predict outcomes accurately. Here, the scalability and quality of the 3D in vitro models become a critical advantage, thanks to several crucial aspects:
- These models can be produced in large quantities, providing the consistent and extensive datasets needed to train machine learning and AI systems effectively. The miniaturization of 3D spheroids allows for up to several million models made from the same donor to harness the biological maturity of primary cells without the effort and inconsistencies of donor changes.
- The high uniformity of size, shape, and cell composition assures training sets for machine learning that truly represent compound effects and tissue response.
- Delivered in high-quality microplates, the 3D tissues can be cultured, treated and handled using standard liquid-handling robotics, without the risk of loss during pipetting steps.
- The plate’s excellent imaging capabilities enable best-in class high-content imaging and cell painting to generate deep data sets – alongside biochemical assays and a range of omics readouts.
- The high scalability and reproducibility of 3D in vitro models paves the way to run studies across broader donor populations, thereby adding patient diversity as an additional data dimension for machine learning.
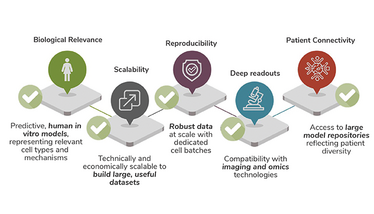
To save the best for the end, the unmet biological and physiological relevance of the data derived from 3D models enhances AI algorithms’ ability to identify novel drug candidates and predict their efficacy and safety profiles with unprecedented accuracy. Only if the training of data sets faithfully represents the biological processes within tissue, and respond to drug challenges correctly, are they suitable for predictive ML.
Before closing, I’d like to emphasize an important point for machine-learning applications of 3D in vitro models: the broad range of biologically relevant readouts, which enables multidimensional data generation. Combining functional biochemical assays, functional imaging assays, cell painting, and a wide range of ‘omics techniques allows the generation of rich data sets to train AI systems. Now, add the capability of probing the response in 3D microtissues generated from different genetic backgrounds, different degrees of disease development, or at different time points and you have a platform that can compile deep and broad data reliably and efficiently thanks to the high scalability of the 3D microtissue approach.
Integrating the right in vitro technologies into an AI-driven drug discovery strategy significantly accelerates the drug development pipeline. AI algorithms can analyze complex datasets from 3D models to identify potential drug candidates and predict outcomes faster than traditional methods. Automated, robust, and scalable workflows are key to generating data sets that matter. The synergy between advanced biological models, high throughput automation, and computational power speeds up the discovery process, reduces the costs associated with drug development by minimizing the reliance on animal testing, and reduces the rate of late-stage failures.
Co-Founder and CEO of InSphero, a renowned biotech enterprise specializing in 3D cell culture technologies. With a Ph.D. from the University of Neuchâtel and extensive experience managing a research group at the Swiss Federal Institute of Technology (ETH), Zurich, Lichtenberg brings unparalleled scientific acumen and visionary leadership to the forefront of pharmaceutical innovation.



















