Oh, What a Wonderful Web We Weave
Could chemically functionalized spider silk be used for innovative drug delivery?
Spider silk has been used in medical settings for millennia – going back to the Ancient Greeks and Romans who applied cobwebs directly to wounds (1). References to spider silk are dotted throughout history and even make an appearance in Shakespeare’s Midsummer Night’s Dream, “I shall desire you of more acquaintance, good Master Cobweb… If I cut my finger, I shall make bold of you.” Scientists have known for some time that some spider silks have antibiotic properties, along with remarkable ductility and tensile strength – comparable with that of high-grade alloy steel.
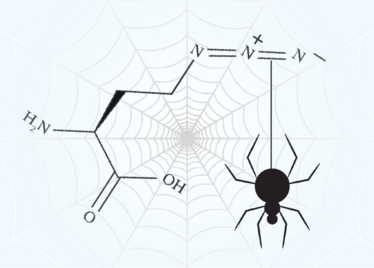
It was these properties that intrigued Neil Thomas, Professor of Chemistry at the University of Nottingham, UK, when he attended a “sandpit” event designed to bring academics together from different disciplines. “There, I met Sara Goodacre [Assistant Professor in the School of Life Sciences at Nottingham] who was talking about the low-level antibiotic properties of natural spider silk,” says Thomas. “I realized that we could produce spider silk based on the ‘4RepCT’ protein – a miniaturized spider silk protein originally developed by a group in Sweden – and modify it to include reactive groups in specific positions, which we could then modify with fluorescent molecules, antibiotics or other drugs.”
The chance meeting five years ago culminated in the publication of a recent paper (2). The group found that the silk could be modified with the unnatural amino acid L-azidohomoalanine – which replaces the L-methionines in the silk proteins – this introduces the azide group which is reactive under very selective conditions without changing its fiber-forming self-assembly properties. In addition, they found that drug molecules or fluorescent molecules could be “clicked” onto the silk proteins either before or after assembling the fibers. If this is done in batches with different antibiotics, the batches of modified silk proteins can then be mixed and self-assembled into fibres decorated with different antibiotics in a known ratio, to help fight antimicrobial resistance where a cocktail of different antibiotics is often used.
“We attached the antibiotic levofloxacin to the silk via a linker with a chemical bond that is sensitive to acidic pH or enzymes released by bacteria,” says Thomas. “As bacteria grow, their local environment becomes slightly more acidic over time, and the chemical bonds between the silk and the antibiotic breaks – slowly releasing the antibiotic and killing the bacteria.”
The researchers envisage the chemically-functionalized spider silk being used to deliver drugs or heal wounds. “We think the main current use, once further testing and optimization has taken place, may be in diabetic ulcers and other slow-healing wounds,” says Thomas.
The silk is generated in bacteria, which has advantages for scale-up and sustainable manufacture. “A recently published pivotal paper (3) from a group working on a similar spider silk system reported being able to spin 1km of silk per liter of bacteria with little additional processing,” says Thomas.
- BM Godfrey, “Traditional and modern biomaterials science uses of spider webs”, (2016). Available at: bit.ly/2iFai6P. Last accessed January 11, 2017.
- D Harvey et al., “Antibiotic spider silk: site-specific functionalization of recombinant spider silk using ‘click’ chemistry”, Adv Mater (2016). PMID: 28028885.
- M Andersson et al., “Biomimetic spinning of artificial spider silk from a chimeric minispidroin”, Nat Chem Bio, 2269, (2017).

Over the course of my Biomedical Sciences degree it dawned on me that my goal of becoming a scientist didn’t quite mesh with my lack of affinity for lab work. Thinking on my decision to pursue biology rather than English at age 15 – despite an aptitude for the latter – I realized that science writing was a way to combine what I loved with what I was good at.
From there I set out to gather as much freelancing experience as I could, spending 2 years developing scientific content for International Innovation, before completing an MSc in Science Communication. After gaining invaluable experience in supporting the communications efforts of CERN and IN-PART, I joined Texere – where I am focused on producing consistently engaging, cutting-edge and innovative content for our specialist audiences around the world.



















