Reverse engineering of a pharmaceutical formulation
The Morphologi G3-ID can be used to generate component-specific particle size and shape data required to aid the reverse engineering of solid oral dose formulations. Here, we provide an example case study for a powder formulation.
Introduction
According to the definition established by the FDA, a generic drug is "a drug product which is comparable to a reference listed drug (RLD) product in dosage form, strength, route of administration, quality, performance characteristics, and intended use". Rigorous rules and regulations pertaining to abbreviated new drug application (ANDA) submissions are complex and the generic drug industry strives to meet these regulations to obtain FDA's approval. And being the "first to file" is the most fundamental principle in the generics business because several companies compete to create generics of successful products going off patent. Therefore, generics companies must be highly skilled and disciplined in product development and achieving bioequivalence-the most critical development area.
Though generics companies commonly use reverse engineering techniques, the topic and, more importantly, the tools needed to carry out the process are rarely discussed in the public domain. In this application note we discuss one such tool that can be used for oral solid dose formulations.
Case Study
Two cold remedy formulations were analysed on the Morphologi G3-ID: one was a commercial brand and the other a generic one. Individual components within the formulations were identified by comparing their Raman spectra with those in a commercial database. Once the components were identified the particle size distributions of individual components in each formulation were compared, as was the overall composition of the two formulations.
Methodology
The cold remedies were dry powder formulations that were automatically dispersed and analysed using the Morphologi G3 -ID. 13 mm3 of sample was dispersed using the instrument's integrated dry powder dispersion unit using the low pressure dispersion option.
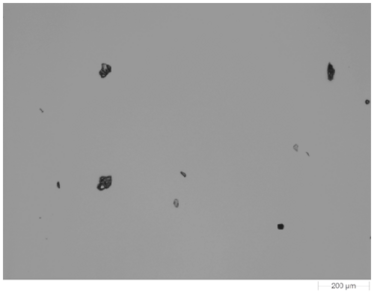
Figure 1 shows an example image of one of the dispersed samples. The samples were morphologically analysed using the 5 x objective. Particles with a circular equivalent diameter (CED) larger than 25 µm were targeted for the Raman chemical identification. In this case the acquisition time was 10 seconds for each particle and spectra from a few thousand particles from each sample were gathered in an overnight analysis.