Some Like it Hot (Melted)
Hot-melt coating may require vast process knowledge and formulation expertise, but when the outcome is reduced processing times and costs, who cares?
Coating has been used in the pharmaceutical industry for decades – initially beginning with sugar coatings. Today, film coating is the most common, with continuous advances being made towards improved environmental protection, aesthetic qualities and more complex drug release profiles. Films coatings generally rely heavily on solvents to dissolve the coating material – and those solvents have a number of drawbacks, including cost and environmental issues. Aqueous coatings are now very popular in the industry but, although they overcome some of the downsides of solvents, they can cause drug stability issues.
An increasing number of companies are turning to novel coating technologies, with one of the most interesting examples being hot-melt coating (HMC). It’s a very clever technology with a simple premise. The API particles are wetted with a molten excipient mix (often including lipids), which is then cooled to form a homogenous coating. The coated API can then easily be combined with excipients like flavors depending on the needs of the patient. Broadly speaking, HMC offers a number of advantages to manufacturers – importantly, no solvent is required and process times are usually short. This results in low energy consumption during production, making HMC a sustainable and ecofriendly technology. It also tends to be very cost effective. All these benefits have been well discussed in literature (1)(2).

The technique has been used outside of the pharma industry for many years, including in the food industry. HMC has a variety of purposes in food, but one interesting use is in coating seasoning mixes used in ready meals. HMC helps minimize hygroscopicity of the seasoning throughout the logistics chain and improve the food’s taste and smell throughout its shelf-life.
The coating challenge
Although the HMC process is fairly straightforward, there are many process input parameters that can lead to unexpected product outputs – and when I first became involved with HMC I discovered there was a lot to learn. The coating process must be performed at a closely controlled temperature, so you need to thoroughly investigate the thermal behavior of your API, excipients, and their interactions. The main drawback of HMC is the amount of formulation and process knowhow required, which is why many companies turn to outsourcing or academic partnerships. For HMC, both the formulation and processing parameters need to be individually optimized depending on the API, which takes time (although such a high level of optimization does bring benefits in terms of intellectual property).
From a chemical point of view, there are almost no limits to the APIs that can be hot-melt coated – it’s even possible to coat thermosensitive APIs – but some physical attributes can cause problems. APIs that are only available as needle-shaped crystals, for example, are almost impossible to coat. At the moment, HMC is very popular for over-the-counter medicines – where usability and sensory aspects, such as taste and odor, can be key market differentiators. Nutraceuticals are also common candidates for HMC, since many vitamins have an unpleasant taste. Increasingly, HMC is being used for oral prescription drugs, including extended, as well as immediate release tablets, and other more complex oral forms, such as orally disintegrating granules (ODGs) – but it’s still early days (and pharma can be slow to embrace change).
Close control
Improved analytical technology has enabled one of the biggest advances in HMC in recent years. For a long time, lipid characterization – essential in the optimization of HMC processes – was expensive and time-consuming, but newer technologies allow us to characterize all polymorphic behaviors of lipids. Process analytical technology (PAT) has also advanced significantly since the US FDA first issued its PAT Guidance for Industry in 2003. Today, state-of-the-art PAT can control manufacturing processes in line, in real time – and be applied to HMC processes. For example, by directly controlling the fluid bed granulator process, inhomogeneities can be identified as they occur. It is also possible to estimate particle size distribution, which helps ensure the quality of the final product. With this type of advanced monitoring technology, you no longer need to perform quality control afterwards.
I believe any development process should be kept as simple as possible. Therefore, it makes sense that companies using HMC technology should ideally opt for granulated or round particles as starting materials. As API particles are often provided in different size distributions, different amounts of lipids are necessary during the coating process; you can measure the thickness of the coating layer during the process using PAT (we use near infrared spectroscopy), modifying the amount of lipids accordingly. The process is easier with a narrow API particle size (optimally 200 to 500 microns) and a lipid with a defined melting point, but other scenarios can work too, with optimization.
It is typically desirable to have almost no crystallographic changes of the lipids during the shelf life of the final product, which can be achieved by using lipids with a specific melting point (rather than a melting range). Though such lipids appear poor from a chemical point of view, they result in stable polymorphs at the end of the process – and that means fewer changes during shelf life. Gaining a solid understanding of the lipid, therefore, is important.
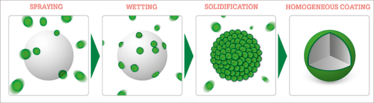
Figure 1. The hot-melt coating process: Molten coating excipients are sprayed onto a seed particle maintained at a lower temperature than the excipient mix. Due to the temperature difference, the droplets attach to the seed particle and solidify upon contact, forming a homogeneous coating.
When it comes to equipment, we use a fluid bed granulator specially designed for HMC. And when choosing a coater, it is important to pay close attention to the air system, which helps improve the reproducibility of the layers on the particles. Once the parameters for the HMC and the excipients mix have been optimized, there are no curing or sintering effects, and very little risk of forming unwanted agglomerates. In addition, the lipid coat can increase the overall hydrophobicity of the final product.
Better medicines
Perhaps most importantly, HMC can help deliver better medicines to patients. Many APIs and pharmaceutical products could benefit from improved taste – and HMC is very effective at taste masking, while also retaining control over the API release profile. Note that the FDA is encouraging developers to pay more attention to what patients want, as it could ultimately improve patient compliance.
What Patients Want
Healthcare companies must pay more attention to the usability and sensory aspects of their products. Patients are increasingly demanding products that are easy to swallow and that taste pleasant. They also want more choice over how they take their medicines – and our research has shown that 50 percent of people in the US have difficulties swallowing tablets or capsules (1).
More complex oral dosage forms, such as orally disintegrating tablets (ODTs) and orally disintegrating granules (ODGs), are starting to emerge – and since they reside in the mouth for much longer than a tablet, a bad-tasting API can be a huge problem, potentially impacting patient compliance. Last year, we used HMC to mask the sour and sulfuric taste and smell of acetylcysteine (NAC). NAC is also a thermosensitive compound – and yet we were able to optimize the HMC process to develop an ODG that had a stable, immediate release profile and unaltered polymorphic form of the coating during storage.
Historically, it has been easier to formulate and produce tablets because the manufacturing processes are well established – and there are certainly many advantages to the humble tablet. Many of these advantages, however, are from a technical rather than a patient point of view. With modern technical capabilities, medicines can – and should – be made more patient friendly. It is now possible to produce almost anything conceivable – and everything is automated. For example, you could make a granule formulation of aspirin taste like cola, if you so desired. If a drug needs to be dosed at a high amount (resulting in a very large tablet), then it makes sense to consider an alternative dosage form. Instant drinks are an effective way of incorporating up to 15 grams of API, and they are becoming increasingly popular. It would be interesting to see more alternative dosage forms, such as instant drinks, ODTs, ODGs and chewable tablets make in-roads into prescription drugs.
Tablets will never be replaced, but patient demands will inevitably lead to an increasing proportion of alternatives.
Reference:
Hermes Pharma, “Market Study Summary: A Hard Truth to Swallow?” (2014). Available at: www.swallowingtablets.com. Last accessed April 26, 2017.
I’ve been working with HMC for years and I’ll admit that the learning curve is steep. It is a specialized technology that is challenging to get up and running, so it’s worth seeking expert advice in the early days. Nevertheless, I expect to see more and more companies adopting HMC for their products – particularly new releases. Who would turn down faster processing (less than two hours to coat a 600 kg batch) and lower costs? So, when making your coating decisions, first consider the advantages – cheaper, quicker and more eco-friendly – for these are particularly compelling in modern manufacturing.
Detlev Haack is Head of Research and Development at Hermes Pharma, Germany.
- SG Sudke, DM Sakarakar, “Hot-Melt Coating: An Innovative Pharmaceutical Coating Technique”, Journal of Pharmaceutical Research & Clinical Practice, 3, 16-26 (2013).
- D Haack, M Koeberle, “Hot Melt Coating for Controlling the Stability, Release Properties and Taste of Solid Oral Dosage Forms”, TechnoPharm, 4, 258-263 (2014).
Detlev Haack is Head of Research and Development at Hermes Pharma, Germany.



















