
Standing up for the Invisible Manufacturers
Despite the increasingly essential role of CDMOs in the pharma and biopharma industry, their collective viewpoints and challenges have been overlooked once too often. Such organizations were in serious need of a voice – and so the Pharma & Biopharma Outsourcing Association (PBOA) was born.
Gil Roth |
Contract manufacturers are unsung heroes. They produce medicines to the same quality standards as pharma companies and comply with the same regulations, but they do not receive the same profits as their pharma counterparts, nor recognition by patients. Their name does not go on the label of the medicines they help develop, and regulators and legislators have tended to overlook the outsourcing sector when making crucial decisions. The issue was highlighted in 2013, with the introduction of Generic Drug User Fee Amendments (GDUFA). These user fees, negotiated by the FDA, the generic industry and several other industry groups, were structured in a way that unintentionally created a large fee burden on contract manufacturing facilities in the generics space. When the sector started to receive invoices from FDA, they wondered why they were bearing this burden when they hadn’t been invited to the negotiating table.
The reaction? The birth of the PBOA. Without an association, things like GDUFA could come up again and, to quote an adage from a senator of Louisiana, “If you’re not at the table you’re probably on the menu…”
Who are you?
It’s difficult to obtain transparency and exact figures about how involved CDMOs are in the pharma industry. Even if you know a facility is making drug X, you won’t know the volumes – and CDMOs and clients treat their projects and partners as heavily guarded trade secrets! According to PBOA’s estimates, CDMOs are involved in around 30 to 40 percent of the drugs made for the US market, but the figure could be higher.
My first encounter with contract manufacturing was back in 1999 when I became the founding editor of an industry magazine called Contract Pharma. In time, I built up a good knowledge and appreciation of the sector, as well as very good relationships with many CDMOs. I started reporting on GDUFA in 2013.
The background to GDUFA is that the volume of Abbreviated New Drug Applications (ANDAs) for generic drugs was increasing and the generic industry wanted the FDA to review these more quickly and work through the extensive backlog of ANDA submissions. Between 2007 and 2011, it was taking the FDA 18 to 30 months to approve an ANDA. There were also delays in foreign inspections. The FDA was keen to accelerate access to generic medicines for patients, but couldn’t expedite the review process without additional funding for more staff and modernized IT systems. Enter generic drug user fees to allow the FDA to build a program to review applications more quickly and effectively, and cope with the challenges of globalization. GDUFA I, as its first five-year authorization period was known, generally mirrored the Prescription Drug User Fee Act (user fees for innovator drugs; introduced in 1992), but where PDUFA had establishment fees that were assigned to the NDA/BLA filers, GDUFA’s facility fees were applied directly to “manufacturing sites” – which meant that CDMOs had to pay out. There were also no reductions or fee waivers for small companies.
User fees are important for the sustainability of the review process, but GDUFA I caused CDMOs many problems. For example, if a facility’s first ANDA was delayed, then CDMOs would end up paying fees – potentially for years – before receiving payment for manufacturing services. CDMOs with multiple sites were hit hard, and some sites were receiving multiple sets of fees because they handled both APIs and finished dosage forms (FDFs). There were also instances of CDMOs receiving bills because out-of-date ANDAs listed their manufacturing sites or ANDA filers listed a CDMO’s site without consulting with the CDMO.
The impact of those FDF facility fees was a case of unintended consequences; GDUFA wasn’t designed to be malicious against CDMOs – those at the table simply didn’t realize how CDMOs would be affected by regulation X or legislation Y because the outsourcing sector did not have representation during negotiations in 2012. A few CDMOs tried to get involved in the negotiating process but were told by the FDA: no trade association, no representation (understandably so, as the FDA would be swamped if they started letting individual companies in).
Once I reported on the impact of the first year of FDF facility fees on CDMOs, I felt very strongly that we needed to do something, so I asked people in the industry if they thought we needed a trade association. The general response was yes – and that I should quit my job at the magazine and run it! I laughed at the time, but soon after the president of one CDMO approached me. He’d been talking with executives at other companies about the need for a trade association, and pushed me to talk with other CDMOs too. At the Contract Pharma annual conference in 2013, I put together a symposium of 30 to 40 representatives from CDMOs. We discussed what the sector wanted and needed from a trade association, and how attendees thought it should be structured and funded. There was a lot of interest, but less idea of what form it would take.
At the time, I didn’t think I was going to leave my job to take the task on, but over the next couple of months, as people kept asking, “What’s next?”, I realized a trade association was never going to materialize unless someone took the lead. I began reaching out more seriously to companies to ask them three basic questions: Do you really believe we need a trade association? Do you really believe I could run it? Would your company put up X amount of dollars as seed money as a founding member? I received a resounding “Yes!” to all three questions from the first five companies that I called. And so I quit my job to launch a trade association.
We All Stand Together
The founding companies of PBOA from 2014:
Sustaining Members
Afton Scientific
Baxter Biopharma Solutions
Coldstream Labs
Cook Pharmica
Gallus Biopharmaceuticals
Halo Pharma
Hospira One2One
Jubilant HollisterStier
Metrics Contract Services
Patheon
Therapure Biomanufacturing
WellSpring Contract Services
General Members
Coating Place, Inc.
DPT/Confab
AAI/CML (now Alcami)
Bringing competitor companies together is always a challenge, and it’s also a challenge to unite companies that perceive themselves as being very different – why should a biopharma CDMO stand with a small-molecule focused CDMO? Ultimately, what dosage form a CDMO is making is less relevant than the fact that they all revolve around providing services. PBOA encourages members to recognize each other as peers. Not all of PBOA’s focus areas will apply to every member (we have a few members who weren’t subject to GDUFA fees at all and we also have a Canadian Affairs Working Group for the 10 member companies that have operations in Canada), but it’s important to recognize that there now exists an association that represents the group as a whole. Through PBOA, CDMOs are taking responsibility for their place in industry. If a member raises something that they feel needs to be discussed – even if it doesn’t apply to every member company – it will gain our attention.
Getting to the table
Progress was fast. Within a week, I’d put together marketing materials, set up meetings, and walked into the Waldorf-Astoria for meetings at DCAT. With a new trade organization, you might have expected it to be a struggle to recruit members, but within two months 15 member companies had signed up. We incorporated PBOA as a non-profit, established a board of trustees drawn from a dozen founder-level companies, and were up and running by the middle of 2014. We held our first board meeting at BIO that year. The goal was clear: to get a seat at the table when GDUFA came up for reauthorization (FDA user fees come up for reauthorization every five years) so that we could negotiate a fee model that offered a more level playing field and was fairer to CDMOs.
Issues That Matter
PBOA has a number of working groups, but it all began with quality metrics. During an FDA public meeting on quality metrics, I asked some questions about the agency’s initial draft guidance during the open comments section. A few of our members were in the audience and afterwards another trade group came by to ask how they could get us involved in their cross-industry group on quality metrics. I told them I would speak with our Quality Technical Group – and then sat down with the members who were in attendance and asked if they would be the core members of our new Quality Technical Group. The group worked with the cross-industry group for a response on the draft guidance, and opted to meet monthly via a conference call to discuss topics coming down the pipe in terms of FDA regulatory dockets, as well as to share questions based on experiences from inspectors and other regulators. They now help members with data integrity questions and promote the sharing of best practices while continuing to address guidances on the FDA’s docket.
We liked the way the Quality Technical Group got our members talking – and I considered it a key success of PBOA. From there, we added a Serialization Working Group, an Over-The-Counter Monograph Group (Congress is currently looking at this space to introduce a new user fee that would primarily be levied on manufacturing facilities), and a Canadian Affairs Working group. We also have an Opioids Working Group. Congress has worked on a number of proposals in the US for dealing with the opioid epidemic, and we want to make sure that our members are providing feedback.
One of our newest working groups focuses on drug shortages – an issue that we’ve been concerned with since PBOA’s inception, and which came into prominence after Hurricane Maria struck in September 2017. Scott Gottlieb tweeted about how FDA is working night and day to help alleviate drug shortages resulting from the disaster in Puerto Rico, which affected many manufacturing plants. I tweeted back that PBOA’s members were ready to help in any way they could to help maintain the supply of critical drugs. A day later, FDA’s Incident Management Group contacted me to ask how our members had been impacted by the disaster.
Supply chains can be very complex and my members’ responses surprised me. I was thinking primarily of companies that operated sites in Puerto Rico, but it turned out some received bioprocessing components from the island and didn’t know if there were secondary sites. Others manufactured product that was shipped to Puerto Rico for the customer, where it was then packaged and sent out globally. We started working with the FDA to fill in the blanks in terms of supply chain, as well as helping companies, such as Patheon, that had facilities in Puerto Rico affected by the storm. From there, we began working on proposal how CDMOs can help when manufacturing site problems lead to drug shortages. The Drug Shortage Working Group has developed a number of interesting ideas on this topic, a sign of the importance of PBOA’s Working Groups.
One of PBOA’s first challenges was to convince the FDA that we were a legitimate stakeholder who should be included in the GDUFA II negotiations. Initially, the FDA seemed a bit unsure about bringing in another trade group. Three trade groups were involved for the first GDUFA, and all were returning for GDUFA II. The FDA didn’t know our sector or how we were different to other interest groups (which underlines how much a trade association was needed). To be taken seriously, we had to be up to speed on many of the policy and implementation issues related to GDUFA I, while making sure that we were protecting the interests of the CDMO sector.
In March 2015, we invited a key FDA policy maker for GDUFA to speak to our members at our Regulatory Workshop in Washington, DC, explain how GDUFA was performing, and to listen to our questions and complaints in terms of the fee structure. A few months later, that policy maker called and agreed that we were an important stakeholder and needed to be involved in the upcoming negotiations.
The actual negotiating period covered 10 months from the fall of 2015 to the summer of 2016. During that time, we tried to make sure that the agreement – and not just the fee structure – would suit both large CDMOs and small CDMOs. In fact, our core negotiating team included two of our largest member companies along with our smallest – to ensure that all voices were heard.
Our efforts paid off. When GDUFA II was signed into law on August 18, 2017, it featured reduced FDF facility fees overall, with a further reduction for CDMO facilities and an exemption for sites that were still waiting for their first approved ANDA. Under GDUFA II, CDMOs now pay one-third the annual FDF facility fee paid by firms that manufacture under ANDAs owned by themselves or their affiliates.
The achievement was proof of concept that PBOA worked and had a strong voice that could impact regulation and legislation. A number of new members joined us after that result. Today, we have 35 member companies (no mean feat given the M&A activity in the sector these past few years). We also have affiliate members, which are companies that provide goods or services, such as marketing, serialization software, and lyophilization equipment, to our members. And we’ve developed a number of working groups where member companies can focus in on key topics (see sidebar, Issues That Matter).
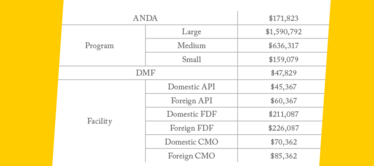
Fees under GDUFA II for 2018.
GDUFA II – Key points
- Facility Fees for Finished Dosage Form (FDF) and API sites account for a smaller portion of GDUFA’s overall collections.
- There are three tiers for the new annual program fee – based on the number of approved ANDAs owned by a firm (20 or more = large; 6 to 19 = medium; 5 or less = small).
- No facility fees for sites identified only in pending submissions; fees are triggered when a site is reference in an approved ANDA.
- No fee for prior approval supplements.
- CDMO FDF facilities will pay one-third of what a non-CDMO FDF facility pays in fees.
- Facilities manufacturing both APIs and finished dosage forms will only pay one fee.
- No CDMO tier for API manufacturing facilities.
- If an ANDA submission is withdrawn before received for filing, the company can apply for a 75 percent refund.
Fees under GDUFA I in 2017:
- Domestic FDF facility: $258,646
- Foreign FDF facility: $273,646
- Domestic API facility: $44,234
- Foreign API facility: $59,234
- Total GDUFA budget: $323,011,000
Facing Congress
The issues with GDUFA I stemmed from the fact that regulators and legislators did not understand the pharma outsourcing sector. Through the process of negotiating GDUFA II, PBOA built relationships with the FDA, not just with the office of generic drugs, but with other departments and offices; awareness of what we do – and the importance of CDMOs – is proliferating to other important offices of the agency. After the GDUFA II negotiations concluded, the FDA contacted us about serialization because the deadlines were approaching and they hadn’t heard directly from CDMOs about their progress. We also had other meetings at the agency, including a sit-down with CDER Director Janet Woodcock and her team to talk about the CDMO sector and what we do, how we can work better with the FDA to help them to better understand new manufacturing technologies, and the role we can play in helping to alleviate drug shortages.
When we first incorporated PBOA, I expected to be dealing solely with the FDA, but I soon learned that once an agreement like GDUFA is made, it is forwarded to Congress, where it is packaged with other bills, voted on, amended, and so on, before ultimately being signed into law by the President. In addition, a lot of FDA initiatives, like serialization/track & trace, originate with Congress. That meant we couldn’t stop at the FDA. We had to begin lobbying Congress to educate them about our sector and to ensure that no one changed the fee model or introduced an “improvement” in GDUFA that would accidentally hurt CDMOs.
This means I had to register as a lobbyist (not something I ever thought would happen, back when I was editing a trade magazine). We also retain an advocacy firm in Washington, DC that helps set up meetings and represents us at Capitol Hill (because outsourcing is good!). Our firm informs us about topics that are coming down the pipe – and there is a lot going on in Congress, especially nowadays, that impacts the overall healthcare sector. Raising the profile of CDMOs has given us the opportunity to meet with the congressional offices, explain the role that CDMOs play, and their economic importance – not just in terms of healthcare, but also in job creation in particular districts and states – and how our members empower biotech startups via development services. Once people understood that CDMOs are a critical (but previously unseen) part of the healthcare ecosystem, they began reaching out to PBOA proactively on new issues and ideas developed by other industry groups to gauge their impact on our members.
I’ve said a lot about FDA and the US Congress, and PBOA is a US-based association, so I am often asked about our relevance to CDMOs outside of the US. Pharma and biopharma is a globalized industry, and if you manufacture for the US market – the biggest pharma market in the world – then, as far as the FDA is concerned, their rules and standards affect you! They will come to inspect you and they will bill you with any relevant user fees. And that means PBOA is the only association that represents you as a CDMO.
We are also expanding our reach – we’re building bridges with Canada’s regulator, as new regulations about foreign facilities could impact the CDMO sector. We’re also talking with a Japanese CDMO association about their needs and interests to see how they dovetail with ours. In time, we’ll look at what we can do in Europe. It’s so important to get our members comfortable with talking to regulators. Regulators are people and they really like to know what’s going on in the industry. I’ve been pleasantly surprised at how interested they are in learning about both the CDMO and business perspectives. They need to know how we differ from pharma companies, even though we are all held to the same quality standards.
PBOA started in 2014 because of GDUFA, but I’m amazed at how far we’ve come and how many other areas of interest we have as an industry. Today we are keeping an eye on many topics from NAFTA (North American Free Trade Agreement), China tariffs and Brexit – which will be very important for our members who sell into the UK and the rest of continental Europe – as well as serialization, opioids, OTC monograph reform, and many others. Drug pricing is another key topic, both on a federal level and within individual states around the country. As talk of new legislation arises, we need to make sure there is an understanding of the difference between pharma companies and CDMOs. If legislation is proposed that says the “drug manufacturer” has to provide X, Y and Z, for example, we would ask for it to be amended to focus on license-holders for drugs, and exempt companies that just perform contract manufacturing. (It is my private mission to get people to differentiate between the license-holder of a drug and the actual manufacturer: a Quixotic quest, at times.)
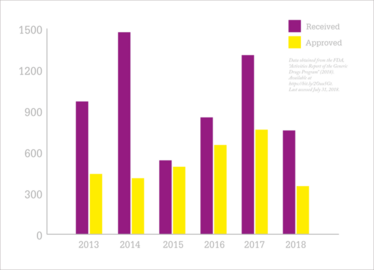
Figure 1: Abbreviated New Drug Applications at the FDA.
Respect
It’s been almost 20 years since I got involved in the sector, and four and a half years since we launched PBOA. During my career, I think clients have garnered a much greater understanding of the importance of CDMOs. Many major pharma companies have drastically re-shaped their supply networks in the last couple of decades and now see their CDMOs as partners (although the definition of “partner” varies wildly). There are many start-ups out there too who are dependent on CDMOs – their own development and commercialization efforts would be so much tougher and require immensely more capital without the availability of contract manufacturers and their development services. At the same time, there is always a need to propagate standardized best practices in terms of how clients and CDMOs work together. There are countless stories from CDMOs about insane client demands – and I’m sure clients have their own frustrations with CDMOs!
I believe CDMOs should be treated with respect, from regulators, legislators, and their customers. It is easy for some people in the industry to dismiss them as service providers, but CDMOs have a huge wealth of manufacturing expertise, are often early adopters of new technology, and are, frankly, indispensable for many pharma and bio companies out there. The collective expertise of PBOA members is outstanding. CDMOs are involved in countless projects and they have seen it all: different drugs, different customers, and different regions. I think it’s fair to say that our members have been exposed to a far wider variety of projects than an in-house pharma site and so they have a great deal to contribute to regulations, legislation, and quality manfacturing.
At industry events, you’ll often find CDMOs standing at booths selling services or waiting for meetings. Rarely are they guests – and even rarer is the conference built specifically for them. PBOA has started running a conference that puts them in the spotlight. I think it shows that we are trying to advance the outsourcing sector – CDMOs are not just sitting idly by, selling services and waiting for things to come down the pipeline; they are discussing the future and how they can prepare for it. We held our first PBOA members conference in October 2017 and the next one will be in September 2018. Along with numerous industry experts, we had five FDA speakers at the 2017 event, covering quality metrics, serialization, mutual recognition of inspections with EMA, inspection trends, and the overhaul of the FDA inspectorate and how it will impact CDMOs. We’ve become a focal point for the FDA to reach out to.
For me personally, it has been a huge learning curve. We have done so much already – but there is still plenty to do. It emphasizes how much a trade association was needed for the sector. We can’t just negotiate one user fee and walk away – we need to make sure that we are invited to the table in the future and have continuity with the FDA and with our industry partners. Every morning I read the Federal Register updates to identify regulations and executive orders that may affect CDMOs (dull reading, but very important!), while our advocacy firm in Washington keeps me apprised of legislative proposals. I send out newsletters to keep our members up to date with what’s going on. We also have monthly legislative update calls for our members, and our working groups meet regularly by teleconference so that members can both learn what’s going on in their areas of interest and relate their experiences. (This has been facilitated by recently bringing aboard Chris Verbicky, one of our first trustees, as PBOA’s Director of Scientific and Regulatory Affairs.)
PBOA exists to make sure that everyone understands the importance of CDMOs, and we will continue to raise the profile of pharma and biopharma outsourcing. We will make sure our unsung heroes are never overlooked again.
Gil Roth is the founder and president of the Pharma & Biopharma Outsourcing Association (PBOA). In 1999, he helped launch Contract Pharma, which swiftly became the premier magazine covering the pharma/biopharma outsourcing and contract services industry. He served as editor of Contract Pharma from October 1999 until February 2014, chronicling the evolution of the pharmaceutical outsourcing sector.
In 2013, while reporting on an article about the Generic Drug User Fee Amendments (GDUFA), Roth had the idea to build a trade association for CMOs and CDMOs, in order to provide them with a voice in regulatory and legislative areas and to raise awareness of the pivotal role they play in healthcare.



















