A Continuous Confidence Boost
Higher productivity, lower hold times, lower costs... Continuous processing of monoclonal antibodies could offer a number of advantages, but regulatory uncertainty and upfront investment remain sticking points. As increasing numbers of case studies emerge, the industry looks set to receive the confidence boost it needs to facilitate widespread adoption.
Gong Wei |
sponsored by Cytiva
In May 2019, Cytiva brought experts together at its Uppsala site in Sweden for “Bioprocess Days.” With a focus on the future of bioprocessing, one key theme of the event was continuous biomanufacturing. Here, we present an article based on an interview with Wei Gong – Director at Henlius Biotech, Shanghai – who presented a case study on “Continuous Manufacture in mAb Production.”
The monoclonal antibody (mAb) field has exploded over the past few years. Case in point; 21 of the 81 mAbs approved since 1986 came in 2017 and 2018. In fact, 49 percent of all global drug sales in 2017 were for antibody drugs – a remarkable statistic. The industry appears to set to continue its upward trajectory, with one analysis projecting a compound annual growth rate of 6.3 percent to reach $131.8 billion by 2023, from $91.1 billion in 2017 (1).
As the biopharmacetuical industry matures, it has been debating which next wave of new technologies will further enhance industrialization of the sector, with the main goals being to improve manufacturing speed, flexibility, and quality, while reducing costs. To that end, several companies, including Henlius, have been evaluating connected and intensified bioprocess steps – and considering how a fully continuous process may look in the future.
Catching up with the curve
Many industries are well ahead of pharma when it comes to adopting continuous processes. In fact, the photo film industry moved from batch mode to continuous production processes in the 1890s! Petrol followed suit in the 1920-40s, and then food in the 1950-80s. For pharma industry, the progress is still ongoing – even for the small molecule sector. For mAbs and other biotherapeutics, continuous processing technologies are very much in their infancy. Noetheless many companies are beginning to see the advantages continuous manufacturing can bring – particularly when it comes to cost savings. For example, by reducing facility and equipment footprints (no hold tanks, smaller bioreactors and chromatography columns) and boosting productivity, continuous processing promises much lower capital costs over the long run. A highly automated system can reduce the amount of operator labor required, and human related errors – both of which feed back into overall cost reductions.
One of the most important advantages of continuous manufacturing is the improved resin capacity utilization with continuous chromatography systems, which leads to lower buffer volumes (and therefore manufacturing costs). We set out to discover whether this would play out in practice by comparing a protein A capture step using batch chromatography system with a periodic counter current chromatography (PCC) continuous set up.
Protein A has grown in step with the antibody market. The first time Protein A was used commercially as a capture step in the purification process was for the first therapeutic antibody, approved by the FDA in 1986 (2). But the Protein A capture step is usually the main cost-driver in downstream processing, with very high attrition costs. A continuous setup involves the sequential use of two columns in a chain such that, as the first column is launched, it is captured by the second one – allowing for continual loading.
With this setup, we found that continuous chromatography increased productivity by 1.5-2.5 times, resin utilization by 1.5 times, while also reducing buffer usage by 60 percent – depending on the residence time. This translates to lower resin consumption, higher productivity and reduced capital investment overall (see box, A Continuous Case Study, for a detailed breakdown).
These results were very promising, but there were a few issues that should be taken into consideration. The first is that there will always be some overload issues in a continuous mode, which may reduces the lifetime of Protein A resin. But we were surprised to find in our lifetime studies that the most important parameter wasn’t the fouling of the Protein A resin, but instead the CIP conditions used. We found that the collected elution amount of mAb from each cycle during overloading of the column only decreased by around 20 percent over 200 cycle resin lifetime, which was very positive. We also looked at impurities and found that remaining host cell protein levels and protein A leakage were consistent over cycles. Overall the quality performance was similar between the continuous mode and to batch mode.
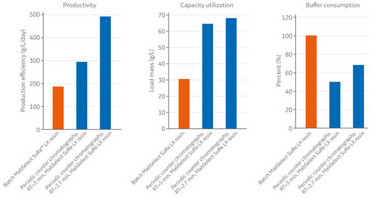
Figure 1. Continuous chromatography increases productivity and resin utilization while saving buffer.
The path to adoption
Despite the strong theoretical basis for adopting a continuous approach to antibody manufacturing – backed by a growing number of case studies demonstrating the practical benefits – adoption is low. A key reason is cost; implementing new technology can be very expensive and few companies want to pay the upfront cost. Another potential barrier is the knowledge required to implement new processes; there are still many questions and a lack of experience with continuous processing as a whole. What should a continuous bioprocess look like? What are the best technologies? And although regulatory agencies have encouraged companies to adopt continuous manufacturing, there are still some details missing in their guidance.
As more companies take the plunge and as we see increasing success stories with continuous manufacturing, confidence will grow and adoption will increase. I see continuous manufacturing as a package of technologies – intimately connected with “industry 4.0” technologies. The current buzz around “digitalization” could help the adoption of continuous manufacturing across the pharma industry (3). For example, creating an effective continuous process is impossible without process analytical technology (PAT). PAT allows for instant feedback and optimization through online sensors, sampling and testing, plus real-time feedback control – it’s like the eyes of a continuous process! Continuous processes will also require automatic control systems, where data is centralized, allowing communication between various machines and improving data integrity. The idea of automated “lights out” manufacturing intelligence will have to be combined with a continuous approach, without manual changeovers and monitoring. In fact, adopting digital technologies are key to successfully achieving continuous production and I see the emergence of both continuous manufacturing and digitalization driving each other forward.
Another crucial factor for the successful implementation of a continuous process is a verification strategy. Quality by Design is essential for continuous manufacturing, as are the control strategy and the viral clearance processes. The downside to a continuous process? Although the risk of a contamination event is lower because there are fewer manual interventions, if a contaminant does get in, it can quickly spread through connected systems. New purification processes will be crucial, such as continuous chromatography (as discussed earlier), single pass ultrafiltration/diafiltration (UF/DF), in-line conditioning, membrane technology and continuous clarification. And, of course, the industry will require regulatory support through additional guidance documents.
With so many companies showing interest in continuous processing, I have no doubt that we will conquer these challenges.
A Continuous Case Study
A more detailed breakdown of Henlius’s continuous chromatography case study.
- For individual columns, cycle time was reduced from 3/4 hours to 1-1.5 hours.
- Load limit increased to 60-80 g/L with continuous manufacturing, compared to 44 g/L with batch mode.
- Production efficiency increased to app. 500 g/L/day when using PCC, compared with just under 300 g/L/day when using the batch MabSelect SuRe LX.
- Capacity utilization increased from just over 30 g/L with batch to over 65 g/L with PCC.
- Buffer consumption increased from 100 percent with batch to as low as 50 percent with PCC.
Read more on continuous manufacturing of mAbs here
- Research and Markets, “Monoclonal Antibodies Market - Forecasts from 2018 to 2023” (2018). Available at: bit.ly/32L4ksE. Last accessed 22 July, 2019.
- B Lain, “Protein A: the life of a disruptive technology”, BioProcess Int., 11, 29–38 (2013).
- Jun Huang, “How Industrial Internet of Things is Transforming Bioprocessing”, The Medicine Maker (2019). Available at: https://bit.ly/33e0HuJ.
Director at Henlius Biotech, China



















