
A Step Change in Pharmaceutical Glass Packaging Innovation
Corning’s analysis into the root cause of glass delamination-related recalls identified boron as the culprit, explaining why process control of borosilicate vials only reduce the risk. As a result, Corning glass scientists developed Valor® Glass, a revolutionary, new aluminosilicate glass composition, developed specifically for pharmaceutical use to eliminate delamination.
sponsored by Corning
Glass is ideally suited for parenteral packaging (1): it’s chemically durable, able to survive high stresses and rapid thermal cycles, it’s transparent, easily sterilized and formable into complex shapes. Historically, the industry used borosilicate glass compositions for sterile injectables due to their hydrolytic performance. But, the borosilicate glasses used for the last 100 years have been the source of numerous issues.
Glass delamination – the appearance of visible flakes or glass lamellae – has contributed to regulatory recalls. Beyond patient risk, delamination can be costly for pharmaceutical manufacturers. A glass supplier for the pharmaceutical industry notes, “For the respective manufacturers, this seemingly minor occurrence can then have costly consequences. One, single recall can cost a pharma company $250M dollars.” (2)
Delamination in pharmaceutical glass has been discussed as early as 1953 (3), but until recently, the root cause of the problem was uncertain. Previously, sterile drug manufacturers didn’t have many options. The industry had to accept delamination as a challenge without a true solution.
Converters of glass tubing to vials attempted to mitigate delamination with process control, which involves monitoring the process to produce vials with smaller heterogeneities (regions of non-uniform glass composition). This approach potentially lessens the risk for delamination; it does not eliminate
the risk.
Corning continues a rich history of product innovation through robust understanding of fundamental materials science. “Several years ago, we were approached by a pharmaceutical company that wanted to understand how and why these defects occurred,” said Dr. Robert A. Schaut, senior research associate, Corning Science & Technology. “This, plus the FDA’s advisory (4) to sterile fill drug manufacturers alerting sterile fill drug manufacturers about the Agency’s concerns with delamination.”
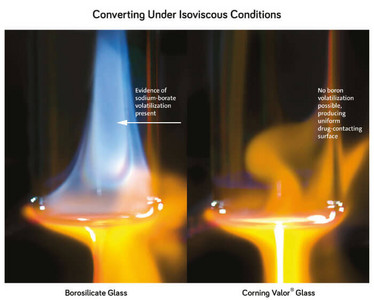
Corning’s analysis included fundamental understanding of the root cause of delamination and inventing a step-change in glass composition to eliminate it. Previous research found that regions of glass contained heterogeneities that were heavily enriched in sodium and boron. Boron is present in borosilicate glass to improve its chemical durability, but the chemistry isn’t uniform because of the tube-to-container converting process. As a result, Corning studied the converting process and how it affects surface chemistry, particularly in regions that are in contact with the drug.
Converting a tube into its final shape includes a series of steps where the tube is exposed to direct flame and then cooled to form the neck, heel and flange of a vial. Type I borosilicate vials include oxides of sodium, boron, and aluminum in the glass network. Sodium and boron become highly volatile (unstable) when subjected to heat, and as the tubing is exposed to direct flame during the converting process, sodium and boron become volatile, evaporating out of the glass network into a gaseous state.
As the vial begins to cool, the sodium and boron deposit onto the sidewall and heel of the vial as sodium borate, and reactively incorporate into the glass surface. As a result, the actual glass chemistry of the vial’s inner-surface is altered, which can increase extractables – and lead to delaminated glass particles – especially from the heel and bottom of the vial that comes into direct contact with a liquid drug.
This explains why enhanced processing techniques to control heterogeneities during converting cannot eliminate delamination. They do not address the root cause – boron evaporation. A carefully controlled-converting process typically relies on an exhaust system to re-distribute volatiles, such as boron and sodium, more homogeneously into the interior of the vial. Factors such as greater wall thickness and vial circumference to heat mean more volatiles per vial, leading to greater propensity for heterogeneous regions in larger vials. And when some of these larger vial formats are analyzed, more delamination is seen. In the end, the risk of delamination cannot be eliminated if the glass formulation contains boron.
Delamination: Eliminated versus Controlled
Based on the root-cause analysis, Corning developed a boron-free glass, while maintaining a glass network comprised of elements used in Type I borosilicate vials, including silica and alumina – Valor® Glass. Corning has shown its aluminosilicate formulation eliminates delamination when compared to borosilicate. We’ve also seen that Valor Glass containers exhibit Type I hydrolytic performance, equivalent or lower extractables concentrations, and suitable drug stability.
“This shouldn’t be surprising given that we haven’t radically altered the formulation of the glass. Really, current glass should be called ‘aluminoborosilicate,’ since they are 70 percent silica and contain both aluminum and boron in modest amounts, said Schaut. “During the development of our aluminosilicate vials, we simply removed boron and adjusted the relative amounts of the other constituents.”
A common question the glass manufacturer hears is, “Your glass is called aluminosilicate; shouldn’t there be more aluminum in the extract?” In fact, high levels of aluminum in the borosilicate extracts are often a result of the heterogeneities ultimately caused by boron. “The amount of aluminum in Valor Glass’ extractable profile is equivalent or lower than with borosilicate alternatives – and is far within safety thresholds,” stated Dan Kramer, development scientist, Corning Science & Technology.
In addition, Valor Glass is designed specifically for pharmaceutical use, based on its extractables performance. Other glass manufacturers have published comparisons of extractables concentrations for type I borosilicate and aluminosilicate glass vials. These comparisons are silent on the actual aluminosilicate glass composition under test. This is important because there are a wide range of glass compositions that fall under this family. To be relevant, it is vital that testing for extractable concentrations use aluminosilicate pedigrees designed, specifically, for pharmaceutical packaging. Otherwise, the results are misleading. Using off-the-shelf aluminosilicate (as intended for handheld electronics) or borosilicate (intended for display applications) to evaluate its fit-for-injectable use is not an appropriate comparison.
“Corning’s aluminosilicate is very different from aluminosilicates already on the market, as the extractables data shows; with the added advantage of eliminating the risk of delamination and associated product recall risk,” said Kramer. “This also means not having to worry about implementing costly measures to control the converting process – Valor Glass can be converted using standard processes and is not vulnerable to delaminate.”
Corning recently published a technical article supporting this in the PDA Letter (5). The data showed Valor Glass has comparable, and even superior, extractable performance when compared to Type I borosilicate glass, including
aluminum extracts.
A step-change in innovation, solving a longstanding problem for the industry
If your glass vial contains sodium and boron, during the converting process, those elements will evaporate from the glass surface, and must travel somewhere.
“You may hope that your process is configured in such a way to reliably remove problematic vials. But you cannot test every vial; there’s no method for assuring each vial produced is truly homogeneous and delamination free – these are sub-microscopic chemical defects that are impossible to screen out,” said Schaut.
There are four steps from the formation of a heterogeneity to delamination. Corning notes those steps include, 1) Formation of heterogeneity, 2) Leaching, 3) Swelling and 4) Spalling off a delaminated flake. Because Valor Glass has uniform surface chemistry and does not form boron-rich heterogeneities during converting, it will not delaminate.
Over the course of the past two decades, the pharmaceutical industry has seen incredible advances in the development of new therapies, as well as in manufacturing technologies and processes. There is also a need for innovation in pharmaceutical glass packaging. The evidence clearly demonstrates Valor Glass represents a significant, and much needed, step forward for glass innovation.
To learn more about this topic, visit Corning’s website
- R Schaut and W Porter Weeks, “Historical review of glasses used for parenteral packaging”, PDA J Pharm Sci Technology, (2017). Available at: journal.pda.org/content/early/2017/02/13/pdajpst.2016.007377.abstract
- Schott, “A new dimension of safety” (2019). Available at: bit.ly/2IAryeY.
- V Dimbleby, “Glass for pharmaceutical purposes”, J Pharm Pharmacol, 5(12), 969-989, (1953).
- FDA, “Advisory to Drug Manufacturers: Formation of Glass Lamellae in Certain Injectable Drugs” (2011). Available at: wayback.archive-it.org/7993/20170113073826/http:/www.fda.gov/Drugs/DrugSafety/ucm248490.htm
- PDA Letter. Extractables Testing of Aluminosilicate and Borosilicate Glass Containers. February 2018. Volume LIV. Issue 2. Available at: www.pda.org/pda-letter-portal/archives/full-article/extractables-testing-of-aluminosilicate-and-borosilicate-glass-containers



















