An Intense Focus on Perfusion
Continuous manufacturing technologies are seeing increased industry uptake, particularly perfusion systems and, increasingly, intensified perfusion. Perfusion offers many benefits to bioprocessing, but choosing the right system and the right medium are key.
Perfusion systems differ from their fed-batch counterparts in that they permit bioreactors to run continuously over extended periods of time by constantly perfusing fresh medium through the culture, simultaneously providing fresh nutrients for the cells and removing waste products. The key advantages of perfusion technology include higher yields per volume, increased flexibility, more consistent product quality and lower capital investment. Furthermore, perfusion systems can be tweaked to support very high cell densities – this “intensified perfusion” can provide even higher product yields. Here, Delia Lyons, Senior Scientist, at MilliporeSigma, describes the benefits of perfusion in modern bioprocessing.
What drew you to this work?
I became interested in cell culture media about 14 years ago, after obtaining a microbiology degree and completing a two-year chemical engineering internship at Massachusetts Institute of Technology. I decided quite early in my career that I wanted to apply my knowledge in cell culture media development and perfusion technology. Today, I lead the Perfusion Media Development Team in the US for MilliporeSigma. We develop both catalogue and customized perfusion media to fit the needs of specific customers.On the one hand we make excellent media for general use, and on the other we fine-tune the media so that our clients can maximize their productivity from a given system.
What big trends are you seeing in bioprocessing?
The growing ability to target disease at the molecular level has resulted in a greater variety of new drugs – many of which address unmet needs. At the same time, processing technologies have evolved to make biomanufacturing more efficient and cost-effective. In particular, I believe that the implementation of single-use systems has been a game-changer – they are fast to deploy and negate the need for expensive and time-consuming cleaning. In terms of business trends, the industry seems to be more competitive than ever before, with many drug companies vying for market share. Patent expiries have depressed prices and there is a growing emphasis on fast-to-market development strategies. In this environment, flexible, cost-effective processes, like perfusion culture, are very attractive to manufacturers.
What is driving the industry’s growing interest in continuous manufacturing?
Fundamental economics is the most important driver – the adoption of continuous processing results in demonstrable savings. Perfusion is one of the leading continuous technologies in terms of adoption in biotechnology. N-1 perfusion saves time and allows you to reduce bioreactor size in the seed train, while production perfusion processing gives much higher protein yields than fed-batch. One of the key advantages with perfusion is its flexibility – the technology is compatible with small, portable plants, and can be used with many drug types over a range of production scales. Also, often perfusion is used with hybrid systems; for example, the combination of fed-batch and perfusion processing.
Perfusion systems can be applied in different ways depending on your objective. For instance, to harvest cells, you may employ a perfusion process in an N-1 bioreactor, or to achieve high-density cryopreservation. Alternatively, if the intent is to harvest protein, you might use a production bioreactor. Generally, production perfusion is interpreted as a process in which the cells are maintained in a steady state, most commonly by active cell bleeding. However, an alternative modality quite commonly used implements a dynamic perfusion in which the cell density is not controlled and viability is allowed to drop similarly to a fed-batch process. Modalities used in the industry for perfusion protein production include microfiltration (or equivalent perfusion systems in which protein is being collected in the harvest), ultrafiltration or hybrid perfusion/fed-batch processes. That said, not all production processes are compatible with perfusion systems at present, so most probably there will always be a need for fed-batch processes. Many companies recognize this by building capacity in both perfusion and fed-batch.
What have been the main technological advances in perfusion systems?
The development of a fully closed upstream system and of more sophisticated on-line and in-line analytical capabilities has made a huge difference to the industry by reducing the risk of losing a perfusion run, which can be costly. In my view, however, the biggest advance has been the introduction of cell separation devices that enable intensified perfusion by sustaining very high cell densities in the bioreactor. That said, there is definitely room for improvement. All current cell retention devices have some drawbacks, even hollow fiber filtration devices (currently the best at providing very high cell densities); for example, some proteins are partially retained by the filter, resulting in lower yields and reduced efficiency. There is a lot of industry interest in developing improved retention devices.
How do perfusion systems help improve product quality?
Perfusion technology allows operators to keep cells in a steady state, providing a uniform and sustained protein quality profile. This, together with the lower protein retention times in the bioreactor, result in better control of potency and immunogenicity. To get the best out of perfusion technology, however, it is very important to choose the right media. Intensified processes give cultures of at least 50 million viable cells per ml, but the cost of medium often prohibits high perfusion rates. If the rate at which we can perfuse the medium is limited, then we should focus on controlling the metabolic profile of the cells, both to minimize toxic by-products and to ensure nutrient levels are adequate for high-cell densities. Such metabolic control can be achieved by fine-tuning the medium according to the cell type and product quality requirements. To increase cell biomass – for example, for cryopreservation, or to inoculate another reactor – extremely high growth and viability rates are required. Conversely, in a production reactor, high growth rates may be unwelcome because keeping cells in steady state would require more medium to be bled off, which again impacts cost of goods. Growth rates can be controlled by careful choice and design of culture medium.
Optimizing media according to the metabolic needs of each specific clone and product and requires significant expertise. In my team, we use a combination of statistical tools and metabolic analysis, as well as the nutritional know-how we have developed over many years. We apply these resources to understand the cells and to then guide them towards customer needs. We have also developed a catalogue CHO cell perfusion medium, which is applicable to a broad range of CHO lines, but can be optimized as necessary.
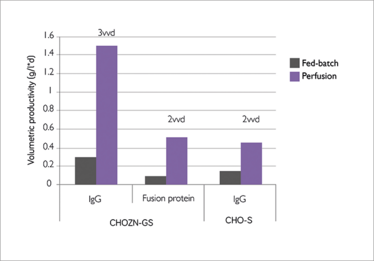
Figure 1. Volumetric production with three cell lines in benchmark fed-batch system compared to perfusion.
What are the challenges of implementing perfusion?
Despite the economic advantages of perfusion, some companies are reluctant to make the transition. Among other reasons, a certain level of expertise is needed to run perfusion systems, perfusion has a higher risk of failure than fed-batch due to more on-line steps, and there is still some fear on being able to demonstrate process control to regulatory agencies. That said, better monitoring and sterility processes have certainly helped make perfusion systems more reliable – and I believe that the benefits are unarguable. However, in a continuous process where cell culture medium is a major cost driver, minimization of perfusion rate is critical, which means the choice of medium is the key to success. Optimized medium will significantly reduce cost of goods; for example our catalogue medium can give more than a five-fold increase in volumetric productivity as compared with fed-batch (Figure 1).
Intensified perfusion is now a reality in bioprocessing; it’s just a matter of time until we see widespread uptake.

For more information about perfusion, please contact [email protected]



















