Considering Capsids
Adeno-associated viruses (AAV) are the most popular viral vectors to date for in vivo applications – but determining full versus empty capsids remains a key challenge
| 5 min read
sponsored by Charles River

Dr. Audrey Chang has always had a passion for driving high quality science in the regulatory arena with a final goal of providing safe and effective biological products to the public. With over 25 years of government and industry experience in conducting biological products testing and in managing laboratories, she has deep interest in helping the industry move forward, including discussing the present future of determining full versus empty capsids remains a key challenge for the AAV-based gene therapy field.
A 2021 FDA Advisory Committee meeting briefing document showed that 24 percent of gene therapy studies worldwide in 2015– 2020 used AAV. Indeed there is a rapidly growing number of clinical investigations and successes – and AAV therapies will undoubtedly move from rare disease indications to more common diseases.
During AAV manufacture, the goal is to produce vectors that contain the therapeutic gene of interest – full capsids, but the process inevitably creates particles that fail to capture the essential genetic information – empty capsids. The percentage of empty capsids can vary from 50–90 percent, and there is also potential for partial sequences or even other variants that package host cell DNA. Right now, the biopharma industry does not fully understand the biology of AAV or what causes the formation of empty capsids – though the manufacturing process certainly plays a role.
So why the concern? Well, empty capsids deliver no therapeutic benefit – and, from a safety perspective, they could increase the risk of heightened immune response from the patient’s total capsid exposure from all these particles. Additionally, the potential of illegitimate packaging of host cell genes can be concerning if they package an antibiotic resistant or oncogene that will unintentionally be delivered to the patient. From a regulatory standpoint, manufacturers are expected to account for empty capsids as a percentage ratio of full:empty particles. This requirement is outlined in guidance from the FDA, but the agency does not call out a specific threshold number. Rather, manufacturers must establish their own critical quality attributes to characterize their product and define risk-based acceptable percentage ratio of full to empty capsids in their batches based on data generated from PD, engineering, and manufacturing runs.
Methods used for testing AAV capsids
As an advanced therapy CDMO leader for over two decades, our expert analytical development services team has first-hand experience testing AAV capsid methods, established and emerging, their advantages and limitations.
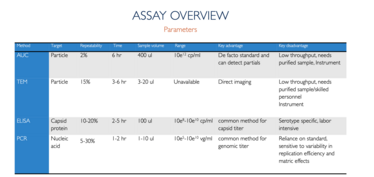
Analytical ultracentrifugation (AUC) is considered the gold standard for determining the percentage of full capsids. In brief, an optical “eye” collects sedimentation velocity profiles over time during the specialized ultracentrifugation run. With this information, it is possible to identify expected peaks of full, empty, and even partially filled peaks. However, it is not a high-throughput method, making it unsuitable for early process development runs or formulation studies.
Transmission electron microscopy (TEM) is a direct visualization method. TEM images – or the newer cryo-TEM images – show empty, full and, to an extent, partially filled populations. But, as with AUC, TEM is not high-throughput and requires specialized instruments and skilled laboratory personnel. It’s also dependent on critical sample preparation steps.
A popular approach for determining full and empty capsids at the lab-scale combines enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) testing. ELISA looks for the capsid protein, which is present on both empty and full populations, whereas PCR only detects particles that contain the genome and can be used to represent the full population. Although both techniques are highly accessible, the data are less than perfect.
Nevertheless, it is common practice to use methods that offer a more rapid turnaround time in the early stages before switching to more established methods for later phase analysis. However, this “switching” can also be problematic if methods and specifications set with the earlier non-GMP methods do not translate in the established method. Additionally, though there are emerging methods1 with promise (1), the burden of comparability studies and, ultimately validation, can be a considerable challenge.
Source: “Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies” Gimpel et al Methods & Clinical Development 20 March 2021
A boost to comparability studies
Given our extensive experience at Charles River with diverse AAV capsid testing methods, we set out to explore the analytical challenges, and conducted a comparison study to evaluate these common techniques used to test capsids. The ultimate study goal was to to share our results with the market, showing the critical role of a reference material for product lifecycle.
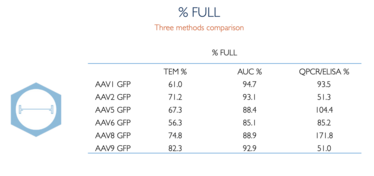
Using the same AAV manufacturing process performed for our clients, we made reference materials – 12 AAV reference material based on six different serotypes: AAV 1,2,5,6,8, and 9 (six full and six empty). Each lot was issued with data generated from a panel of QC analytical assays, including genomic copies, pH, bioburden, mycoplasma, endotoxin, purity, and percentage of full/empty capsid ratio by TEM.
With our reference materials, we performed a side-by-side comparison of the percentage of full/empty capsids with TEM, AUC and PCR/ELISA. The AUC percentage full numbers for AAV reference material were higher in value compared with TEM – and also closer in agreement than to PCR/ELISA. PCR/ELISA showed much higher variability and less reliability – not unexpected given that these methods are indirect.
Our comparison study really showcases how reference materials can be used to demonstrate comparability between different methods. Having a reference standard and/or reference material enables the translation of data from development, comparability studies, validation/ revalidation, technical transfer, and assay trending. Reference standards also allow for well-designed bridging studies for future versions of the process. And so you may be pleased to know that we’ve made these reference materials for AAV 1,2,5,6,8, and 9 (six full and six empty) available to our clients to help generate data for both today’s and tomorrow’s analytics.
To learn more about the study, watch the on-demand webinar available here: Empty vs. Full AAV Capsid Analysis: A Comparative Study Using AAV Reference Materials.
For more information on Charles River’s AAV products, please visit our website
- AL Gimpel et al., Molecular Therapy: Methods and Clinical Development, 20, 740-754 (2021). DOI: 10.1016/j.omtm.2021.02.010
- AK Werle et al., Molecular Therapy: Methods and Clinical Development, 23, 254-262 (2021). DOI: 10.1016/j.omtm.2021.08.009
- Yang et al Gen BioTechnology (2022)



















