Driving Down Biosimilar COGS
To keep biosimilars costs as low as possible, take a good look at your purification and characterization processes
Amanda Turner, Khaled Mriziq | | Quick Read
The expensive, specialist methodologies that underpin the development and manufacture of important biological medicines typically result in associated costs being passed to healthcare providers, insurers, or the patients themselves. Biosimilars, therefore, are welcome players in the market. As originator therapies reach patent expiry, biosimilars aim to deliver on the promise of greater affordability and wider access to biological therapies. However, the world of biosimilars is exceptionally competitive – with many biopharma manufacturers racing to become the first to leverage the opportunity of blockbuster exclusivity loss.
Although biosimilars must only demonstrate equivalence to the originator/reference product to gain regulatory approval, standards remain rigorous, and interchangeability with the reference product needs to be shown regarding molecular structure, biological activity and efficacy, safety, and immunogenicity. Purification and bioanalytical characterization processes can comprise more than half of the total development cost for a biosimilar (1). In our view, upfront planning and access to the most effective protein purification and bioanalytical tools are vital to reduce the risk of failure, maximize the chances of timely regulatory approval, and ensure long-term drug quality during large-scale manufacture – and beyond.
Purification and recovery methods must achieve high protein purity and yield – but by cost-effective means, as costs need to be kept as low as possible to compete effectively in the biosimilars market. Removal of impurities, including host cell protein, DNA, viral contaminants, protein aggregates, isoforms, and other species, should use high specificity chromatography technologies that are able to withstand elevated throughput rates and variable pH conditions. The traditionally favored Protein A resins provide excellent specificity but are an expensive option. Alternative high-capacity resins, on the other hand, can offer opportunities to lower expenditure while also optimizing purification processes (2) (3) (4); for example, ion exchange resins have demonstrated comparable results to Protein A-based processes regarding the efficient clearing of impurities with good binding capacity and stability – and without the limitations of flow rate or pH conditions (3). Mixed-mode chromatography resins are unique in their ability to combine varying forms of molecular interaction (for example, hydrophobic, ion exchange) via a single-support matrix, and can reduce the number of purification steps required in some cases (4). Integration of such technologies within existing pathways allows efficiencies to be made with minimal disruption to the development plan.
Purification optimized? Check.
Next, let’s look at the comparative clinical studies needed to determine pharmacokinetic and immunogenicity profiles of the reference product and biosimilar. You’ll need to develop sensitive and selective ligand binding assays using specialized antibody reagents. Notably, the reliability and reproducibility of the data generated are contingent on the quality of the antibody reagents selected, so securing high quality, reproducible antibodies early in the development lifecycle is beneficial.
When the biologic is a monoclonal antibody, anti-idiotypic antibody reagents are critical for bioanalytical assays comparing biosimilar and reference product functionality. In vitro antibody generation methods (for example, antibody phage display) can selectively produce reagents demonstrating high specificity for defined regions of the drug, allowing assays to be designed to detect free drug, total drug, or the drug-target complex. In vitro technology offers the benefit of antibody generation within three months, while traditional animal immunization methodologies can be slow (approximately six to nine months) and may not result in the desired level of specificity. Recombinant antibodies are sequence-defined from the outset and well characterized, permitting an indefinite supply of reproducible capture and detection reagents. Through antibody engineering, technologies can be incorporated that enable site-directed conjugation and fast assembly of antibodies in monovalent or bivalent Fab and full-length immunoglobulin formats (for example, SpyTag-SpyCatcher technology) (5). Such innovations enable tighter control of critical reagents and speed up the assay design and optimization process, resulting in more sensitive and robust assays.
As with all biological products made in cellular systems, small molecular changes may arise between biosimilar batches and alterations are introduced over time as the manufacturing system evolves. Reliable bioanalytical assays are vital in demonstrating that molecular modifications do not deleteriously affect drug efficacy or safety, and in ensuring the success of the drug – long after regulatory approval.
As an increasing number of biopharmaceutical developers seek to maximize the opportunity of biosimilar medicines, robust and accelerated approaches to bioprocessing and bioanalytical data generation are becoming increasingly important for success. In our view, those companies that embrace technologies to enhance the efficiency and quality of their methodologies are most likely to avoid regulatory setbacks – and thrive in this hugely competitive marketplace.
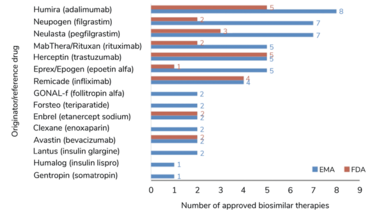
Originator therapies with associated biosimilars currently approved by the EMA and FDA. Adapted from Gherghescu et al. (6).
Headshot and figure supplied by company
- “BJ Gutierrez, “Financial Analysis of Biosimilar Development Candidates: A Case Study on the US Biosimilar Business,” Master thesis, Harvard Extension School (2015).
- DT Ta et al., “A new and simplified anion exchange chromatographic process for the purification of cell-grown influenza A H1N1 virus,” Separation and Purification Technology, 263, 118412 (2021).
- Bio-Rad, “A Blueprint for Robust, Cost-Efficient Biosimilar Purification Using Ion Exchange Resins,” (2021). Available at https://bit.ly/3jVxlvS.
- Bio-Rad, “Bulletin 6895: Novel resin functionalities maximize process value for biomolecule purifications,” Available at: https://bit.ly/2Xd7ibt.
- AH Keeble et al., “Approaching infinite affinity through engineering of peptide-protein interaction,” Proc. Natl. Acad. Sci. USA, 52, 26523-26533 (2019).
- I Gherghescu et al., “The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA”. Pharmaceutics, 13, 48 (2021).
Amanda Turner is Senior Product Manager, Custom Antibody Products, at Bio-Rad Laboratories, CA, US
Khaled Mriziq is Senior Global Marketing Manager, Process Chromatography, Protein Purification Group, at Bio-Rad Laboratories, CA, US



















