Flow Chemistry Focus
Professor Steven Ley runs a research group at the Whiffen and Innovative Technology Centre in the Department of Chemistry, University of Cambridge, UK – and a significant focus of the group’s work right now is on flow chemistry and organic synthesis. We catch up with Daniel Fitzpatrick, a PhD student with the group, to find out how the group is working to synthesize a range of APIs on a single reactor platform that uses flow chemistry and a clever cloud-based system.
What made you choose to work in the area of flow chemistry and with Steve Ley?
My background is in chemical engineering and I wanted to apply principles from this area directly into pharmaceutical development. Ideally, I want to help bring the benefits of continuous processing, which have been around for a very long time in the petrochemical industry, into drug synthesis.
Back in 2013 when I first joined the group, flow chemistry wasn’t a huge area of research (growing, certainly, but not as large as it is now) so I thought it would be a great time to get involved. Steve’s group is very much at the forefront of flow chemistry so I got in touch with him to ask if he’d take me on as a PhD student – and fortunately he said yes! I’ve been here ever since.
What does the group focus on?
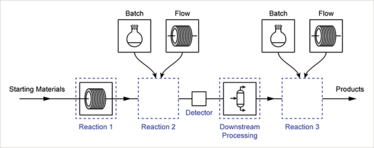
The Ley Group performs a lot of research in the area of integrating enabling tools into synthetic processes. One large area of this is the development of new flow chemistry tools – something that lends itself very well to pharmaceutical development, from discovery all the way through to process scale-up and manufacturing. We develop new techniques in-house to solve problems we encounter, mainly at an R&D level, but we also work closely with equipment manufacturers to test and help develop new tools.
Our philosophy is not one where we want everyone to use flow for all processes, but rather one where we want to develop new tools and techniques to such a level that chemists can choose the best method for whatever they are hoping to achieve – whether that be a batch process or a flow process. You can read more about how we enable this integration of batch and flow processes here: http://rsc.li/2nEdPYp.
How are you working to develop a portable device for synthesizing medicine?
My work ties in directly with the computer automation side of things; for example, how we can control and monitor synthesis processes as they are running. We have developed a cloud-based system that can connect with any piece of lab equipment we have and use data collected to monitor process operations to, for instance, check for steady state, or to monitor for safety purposes. This system requires very little in terms of computer hardware and can run on a Raspberry Pi computer (very cheap, and roughly the size of a mobile phone). It’s ideal for integrating into a miniaturized synthesis system. We can also control equipment for automation purposes, such as system start up.
We are now working on a project to synthesize a range of APIs on a single reactor platform using this control system, highlighting the versatility of flow chemistry and its ability to synthesize a range of molecules quickly and with minimal changeover of reactor components.
What are the benefits of using flow chemistry compared with traditional pharmaceutical manufacturing?
There are many! The main ones are:
- It’s much easier to automate processes.
- Processes can be telescoped (read more at: http://tmm.txp.to/0317wiley).
- Scale-up from discovery level to process development to manufacturing is much easier.
- Once a system reaches steady state, all you need to do is ensure that reservoirs of feed material stay topped up, and the system will continuously pump your product out the other end of the reactor.
What are the challenges of making flow chemistry portable?
One big challenge is the ability for the system to cope with instability. If something goes wrong, it needs to be easy to fix or replace parts internally in the system. This is especially the case for the type of portable unit that DARPA has in mind, which needs to be taken to a war or disaster zone. In addition, measuring the purity of the drug compounds that are produced is a challenge in a portable unit. While spectrometers such as nuclear magnetic resonance and infrared units are getting smaller, they are still sizable and can be heavy, which makes them difficult to fit into small spaces.
What other projects are you involved with?
I’m currently running a project to accelerate the drug development process, after a target compound has been identified. This project involves self-optimization (where the control system finds optimal reaction conditions with minimal human intervention) and reaction telescoping to give a steady stream of final API from raw starting materials in as short a time as possible.



















