How Green is Green?
The field of green chemistry is concerned with minimization of waste and avoidance of toxic and hazardous substances in pharmaceutical products.
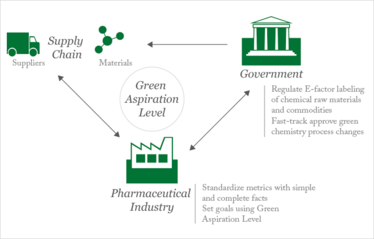
Figure reproduced from (5) by permission of The Royal Society of Chemistry.
The rapidly evolving field of green chemistry is concerned with minimization of waste and avoidance of toxic and hazardous substances in the production and application of chemical and pharmaceutical products. Why should the pharmaceutical industry in particular be interested in promoting green chemistry? We believe that the broad adoption of green chemistry principles as a core business strategy will increase the industry’s sustainability and R&D productivity (1), and also bolster its reputation (2), in the context of rising healthcare costs combined with growing public awareness of high-profile environmental issues like global warming.
Strides have been made within the pharmaceutical industry to incorporate potentially disruptive green chemistry and engineering philosophies over the past two decades since the invention of the E-factor (3) and introduction of “The 12 Principles of Green Chemistry” (4), yet there are still a number of barriers to full adoption.
There is an old management adage that states, “You can’t manage what you don’t measure”, and unfortunately the science of green chemistry does not have a standard measure for process ‘greenness.’ Instead, there has been a proliferation of green chemistry metrics over the past decade. E-factor and Process Mass Intensity are frontrunners, but no clear favorite has emerged. As such, green chemistry is perceived by some as a scientific curiosity with many ‘soft’ and non-quantifiable descriptors.
Another significant impediment to broad adoption of green chemistry is that we lack a clear definition of process starting points in the assessment of process greenness for the synthesis of active pharmaceutical ingredients (APIs). This has been a bothersome source of inconsistency in the literature. Failure to define an appropriate starting point can lead to exclusion of varying amounts of intrinsic raw material waste created during earlier stages of production.
We must also consider that current measures of process greenness do not explicitly account for complexity of the target molecule and availability of technology to make it. This is important – APIs are not all created equal, and their manufacturing process complexities vary greatly.
Lastly, pharmaceutical firms have little incentive to develop ‘optimally green’ manufacturing processes due to high project attrition rates during development, limited patent lives of medicines, and perceived regulatory risks associated with the filing of greener and more economical second-generation processes.
In an attempt to tackle some of the barriers to green chemistry, we recently created the Green Aspiration Level (GAL) concept as a metric to quantify the environmental impact of producing a specific pharmaceutical agent, while taking into account the molecular complexity of the API (5). GAL also clearly defines process starting points, and utilizes the complete E-factor as the mathematically simplest concept to derive the amount of chemical waste generated from the entire manufacturing process. In addition, GAL utilizes process waste data generated by the American Chemical Society Green Chemistry Pharmaceutical Roundtable (ACS GCI PR) to define average industry process performance as a reference point of comparison (6). Though the science of measuring molecular complexity is still in its early stages, GAL also took advantage of Baran’s simple yet effective process ideality methodology, which represents both molecular complexity and the degree of optimal implementation of available chemical technology (7).
The result? GAL allows, for the first time, the objective assessment of green process performance relative to industry peers, thus giving scientists and managers a much-needed tool for measurement. Since it is quantifiable, GAL will also allow governments to sponsor green pharmaceutical manufacturing initiatives by considering incentives such as fast-track regulatory approvals for green processes, and regulations such as labeling of commercially available raw materials with complete E-factors.
We believe GAL can play a critical role in breaking down the barriers to broad adoption of green chemistry in the pharmaceutical industry. A critical first step is to get buy-in from the industrial community, which may best be achieved through dialogue within collaborative pharmaceutical consortia, such as the International Consortium for Innovation & Quality in Pharmaceutical Development and the ACS GCI PR. With their help, we could implement the new measuring tool across the industry, facilitate communication with governments to foster green chemistry initiatives through regulations and incentives, and make green chemistry part of national conversations about public policy.
Frank Roschangar is Director, Process Research & Global External Chemistry Management Chemical Development US, and Chris H. Senanayake is Vice President of Chemical Development US at Boehringer Ingelheim, Ridgefield, CT, USA.
G Alan Kurose is President and CEO at Coastal Medical, Providence, RI.
Roger A. Sheldon is Professor (Emeritus) of Biocatalysis & Organic Chemistry at Delft University of Technology, The Netherlands.
- S.M. Paul et al., “How to Improve R&D Productivity: the Pharmaceutical Industry's Grand Challenge”, Nature Reviews Drug Discovery 9, 203-214 (2010).
- J. LaMattina, “When It Comes To Its Reputation, The Pharmaceutical Industry Continues To Mess Up", Forbes.com (24 Sep 2014), www.forbes.com
- R.A. Sheldon, “Organic Synthesis; Past, Present and Future.” Chem. Ind. (London), 903-906 (1992).
- P.T. Anastas and J.C. Warner, “Green Chemistry: Theory and Practice.” Oxford University Press (1998).
- F. Roschangar, R.A. Sheldon and C.H. Senanayake, “Overcoming Barriers to Green Chemistry in the Pharmaceutical Industry – the Green Aspiration Level™ Concept", Green Chem. Advance Article, doi: 10.1039/C4GC01563K (2015).



















