How to Beat the Bottleneck
As the accelerating number of monoclonal antibodies on the market continues to drive higher upstream titers, chromatography resin suppliers must continually strive to improve productivity, quality and overall process economy.
sponsored by Cytiva
The monoclonal antibody (mAb) field has come a long way since the first therapeutic mAb was commercialized in 1986. As of today, over 60 mAbs have been approved in the US and Europe and sales of mAbs are expected to cross $125 billion by 2020 (1). As the number of mAbs on the market and in development has accelerated over the years, so too have upstream titers. With the increasing upstream titers, the constraints on downstream processing have only increased. The efficient recovery and purification of mAbs from cell culture medium is a critical part of the production process and can contribute significantly to the total manufacturing costs (2). Protein A affinity chromatography is commonly used in the process-scale purification of mAbs because it is an easy, fast and selective procedure for capturing the target protein – often resulting in a greater than 99 percent purity from complex cell culture media in a single step.
The demands of protein A resins have evolved significantly over the years and the nature of the process means that the capture step can act as a bottleneck – especially as upstream titers continue to increase. I believe suppliers must do what they can to keep pace with the constantly evolving demands of the industry. And this is why Cytiva has made the long-term strategic decision to constantly improve our protein A chromatography resins.


Native protein A chromatography resin first began seeing routine use in the lab in the 1980s, but approximately every five years since then there has been a new demand from the industry for further innovation – for resins with new properties to address new challenges. One of the early developments was the switch to recombinant protein A, as the industry moved towards animal-free products. Then, as production scales continued to rise in the early 2000s, we introduced the “second generation” in protein A resins, the MabSelect resin, the first in a family of products, to allow for higher throughput downstream processing.
These resins are based on a highly cross-linked agarose matrix with a recombinant protein A ligand. In 2005, GE launched the MabSelect SuRe resin that has an alkaline-stabilized protein A ligand, which allows for efficient cleaning and sanitization with reagents such as sodium hydroxide (NaOH), eliminating the need for expensive and hazardous cleaning agents. Six years later, we launched MabSelect SuRe LX to meet the needs associated with high-titer upstream processes. One of the key features of MabSelect SuRe LX is its increased dynamic binding capacity (DBC), which allowed better economies of scale. More recently, we’ve worked to improve the capacity of our protein A chromatography resins to address the industry shift towards continuous manufacturing. MabSelect SuRe pcc further increases capacity at short residence time, making it well-suited for applications requiring fast mass transfer as seen in continuous processes.
Moving forward
The biopharma industry continues to face new challenges, so it is incredibly important to continue to innovate and develop new solutions to support the market. Throughout the evolution of our protein A resins, the need for greater productivity has been a constant theme, and never more so than today. That’s why our latest solution, MabSelect PrismA, is designed to achieve high capacity to increase mass throughput, enabling greater productivity of current chromatography columns and systems to be improved without costly capital expenditures (see the infographic for more details). With increased binding capacity, MabSelect PrismA allows for up to 30 percent more target mAb to be purified using current equipment; alternatively, the resin volume required to achieve a given mass throughput can be reduced.
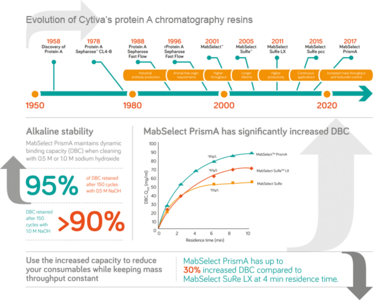
MabSelect PrismA also seeks to address bioburden challenges with improved alkaline stability to enable more efficient cleaning and prevent growth of microorganisms and inactivate potential endotoxins. Protein A columns are more prone to contamination because of heavy impurity load and weak tolerance for common concentrations of NaOH cleaning-in-place solutions. Regulators are therefore increasingly asking manufacturers to control the sources of bioburden. Improving tolerance for NaOH will continue to pose a significant challenge for the industry in the future.
As we look ahead, it seems likely that upstream titers in mAb production will continue an upward trend. The protein A capture step does create a production bottleneck in downstream processing and it remains to be seen whether current technologies can keep pace. Another major challenge for the field is the development of next-generation antibody constructs. The industry is rapidly moving beyond conventional mAbs towards a variety of new constructs, including antibody fragments, Fc fusion proteins, bispecific antibodies and antibody-fusion proteins. These new antibody variants most often require modifications of the original mAb platform process to enable production. There might also be the potential to modify protein A to create a tailored version based on antibody structure.
Today, we’re excited to be talking about our latest chromatography resin, MabsSelect PrismA, but there is always room for further innovation and I have no doubt that we will be sharing the next step in protein A evolution and other affinity chromatography solutions down the road.
Kajsa Stridsberg-Fridén is Senior Project Manager R&D at Cytiva, Sweden, and has been with the company for more than 15 years. In her current role, Kajsa is managing cross-functional projects, developing chromatography products for the biopharmaceutical market.
- AA Shukla et al., “Downstream processing of monoclonal antibodies - Application of platform approaches”, J Chromatogr B, 848, 28–39 (2007). PMID: 17046339.
- AA Shukla et al., “Evolving trends in mAb production processes”, Bioeng & Transl Med, 2, 58–69 (2017). PMID: 29313024.



















