How to Embed Excellence
Having witnessed the evolution of operational excellence (OPEX), how do you implement it – and how do you ensure continued success? Here, we propose a new model.
In our previous article in The Medicine Maker (1), we traced the evolution of Operational Excellence (OPEX), from increasing regulatory pressure in the early 2000s for more transparent manufacturing processes, to today’s widespread adoption of OPEX principles. Most pharmaceutical plants in both Europe and the US have been running operational improvement programs for years. But the journey to excellence is a never-ending one if you want to maintain success.

In 2014, with our St. Gallen OPEX benchmarking project in its tenth year and 277 data sets in our database (2), we felt the time was right to further develop our ideas around the future of OPEX. In particular, we wanted to align our research with the current needs of the industry. With this in mind, we launched a new global research collaboration with four of the world’s biggest pharmaceutical companies. We held a series of meetings, both on neutral ground and ‘amidst the action’ at manufacturing sites, to discuss the current challenges of the industry and how OPEX could be better implemented.
Our participants all faced similar challenges and a number of questions kept coming up in workshop discussions. How do we embed an OPEX program over time? How do we maintain a successful OPEX program? And how do we measure progress? These FAQs led us to the idea of an OPEX maturity model that could be used to assess OPEX performance on site. Figure 1 shows the first draft of the model and recommends an order for tackling different aspects. Here, we will introduce the key parts of our model, define the individual maturity stages and explain how the level of maturity of a site is assessed. Let’s start by describing each step of OPEX implementation.
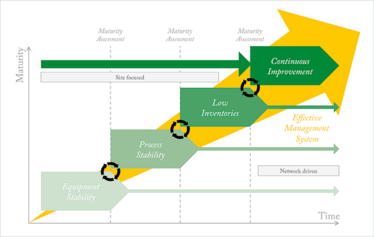
Figure 1: The St. Gallen Operational Excellence Maturity Model. A suggested order for implementing the different stages of OPEX and assessing performance.
Step 1: Equipment Stability
The goal of this first maturity level is to achieve highly stable manufacturing equipment – after all, it is impossible to create robust processes without reliable equipment. A production site can be said to have achieved Equipment Stability when there is a genuine sense of urgency to maximize equipment effectiveness and improve maintenance efficiency. As equipment breakdowns can lead to production downtime and bring about a crisis, the concept of total productive maintenance, in which all workers learn how to clean, inspect and maintain equipment, is something that we believe is crucial in the quest for an excellent production environment.
Step 2: Process Stability
Process Stability is achieved when a manufacturing plant has internalized the principles of total quality management, which involves continuously isolating variables that cause deviation, mastering them and, by doing so, steadily improving their processes in the direction of flow. A special emphasis on variance minimization leads to a more stable and better-controlled manufacturing process, which in turn reduces the need for safety stock to act as a buffer. Moreover, the plant ensures that supplier quality management is up to the same standard by integrating suppliers into the internal quality system.
Step 3: Low Inventories
The third step is to reduce operating inventory to increase flexibility and responsiveness. Following the just-in-time (JIT) principle, the site produces what is needed on receipt of a signal from the customer. With the goal of one-piece flow (continuous manufacturing) and minimal buffer inventory, it’s obvious that this maturity level requires stable equipment and robust processes; therefore, the first two levels must have been mastered first, if you don’t want the danger of the whole underlying system starting to crash.
Step 4: Continuous Improvement
Step 4 is the end goal but also a moving target given its name. It is the stage at which we can truly say that we have achieved OPEX. It is reached when all the requirements of each maturity level have been met, with appropriate practices put in place. However, the journey should continue with a commitment to ongoing efforts to stay competitive by improving products, services and processes, and by collaborating with suppliers and customers to share the benefits.
Stability Enablers
Companies who agree with the following statements are more likely to achieve stability.
- In our company direct and indirect processes are well documented.
- We continuously measure the quality of our processes by using process measures (e.g., on-time-in-full delivery rate).
- Our process measures are directly linked to our plant objectives.
- In our company there are dedicated process owners who are responsible for planning, management and improvement of their processes.
- A large percentage of equipment on the shop floor is currently under statistical process control (SPC).
- We make use of statistical process control to reduce variances in processes.
- For root cause analysis we have standardized tools to get a deeper understanding of the influencing factors.
These factors had a statistically significant impact on the following KPIs
- Changeover time
- Production schedule accuracy
- Unplanned maintenance
Assessing Progress
For each maturity level there is a set of requirements that have to be met, and achieving these means applying a variety of practices. Measures put in place by the site leadership team don’t need to be complex and in many cases a single process or program may fulfil the intent of one or more requirements. For example, simple housekeeping activities like maintaining a checklist to continuously monitor the condition and cleanliness of the machines and equipment has a high impact on equipment stability but also on the overall quality management of the plant. On the other hand, sometimes meeting a single requirement may take multiple efforts. For example, to achieve a high level of equipment effectiveness, there are several activities that have to be put in place by the site leadership team, including deploying a formal program for maintaining the machines and equipment, identifying all potential bottleneck machines and supplying additional spare parts, as well as emphasizing good maintenance as a strategy for increasing quality and planning for compliance. Strong management commitment will also be required to provide support in the form of playbooks, methodologies, coaching, and training and certification of staff.
Maturity level assessments should be conducted to validate site performance and are an effective and consistent way of measuring a site’s progress along the pathway to OPEX. The assessments, developed by the University of St. Gallen, are designed to allow production sites to verify whether they have reached the next maturity level. The assessment, which is carried out by the companies in collaboration with the University, occurs when the site leadership team believes the site has completed the requirements for the next level. The scope of the maturity level assessment includes:
- verification of performance results
- verification of required practices
- review of the diagnostic process and action plans
The assessments should be as frank and open as possible to avoid misunderstandings and misleading implications. The best approach is to use multiple assessments by different people from different departments. One person should consolidate the findings and be responsible for feedback and follow up to ensure the quality of the data.
If the site meets the requirements for the next maturity level, the actions are submitted as shared practices for the entire network as one of the outputs from the maturity level assessment. The output form of a (failed) maturity level assessment includes an action plan to fulfill any requirements that have not been met (including root cause analysis).
The basic ideas we developed and discussed in our workshops were also backed up with data from the St. Gallen OPEX database, which allowed us to analyze the correlation between excellent performance in the chosen key performance indicators (KPIs) and specific enabling factors. Figure 2 shows how we define excellence in a range of KPIs. The numbers in Figure 2 are based on the St. Gallen OPEX database. The excellence scores were derived by using the top ten percent of the included sites, while the low performance scores relate to the bottom ten percent of the sites that participated in the St. Gallen OPEX Benchmarking. “Stability Enablers” shows the enablers that support strong performance in the individual categories. The results of the statistical analyses show that companies who implement these measures have higher performance - they are the key to achieve a superior maturity level.
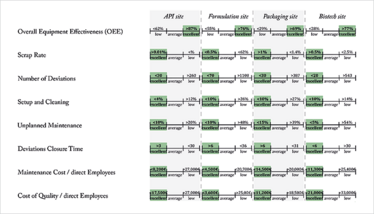
Figure 2: Low, average and excellent performers in the individual performance categories.
We hope that our Maturity Model will help you to measure your OPEX success and ultimately help drive the industry as a whole on its never-ending journey to excellence. Working with industry and conducting the workshops was incredibly valuable for us and we plan to continue this exchange platform in 2015 to ensure that the future of OPEX remains bright.
- T. Friedli, C. Mänder and P. Basu, “The Evolution of Operational Excellence”, The Medicine Maker 2, 30 (2014).
- T. Friedli et al., “Leading Pharmaceutical Operational Excellence”, Springer Verlag, Berlin (2013).
- T. Friedli et al., “Operational Excellence in the Pharmaceutical Industry”, Editio Cantor Verlag, Aulendorf, Germany (2006)
Thomas Friedli is a researcher at the University of St.Gallen, Switzerland.
Christian Mänder is a researcher at the University of St.Gallen, Switzerland.
Nicolas Ponce is a Research Associates, at the University of St Gallen Institute of Technology Management.



















