
It’s a Kind of (Formulation) Magic
Meet the grand winner of The Medicine Maker 2018 Innovation Awards: Zydis Ultra, an orally disintegrating tablet made using resonance acoustic mixing that aims to boost patient compliance and acceptability.
Zydis technology – the first orally disintegrating tablet (ODT) – was commercially developed by Catalent in the 1980s. As the name suggests, such tablets are specifically designed to disintegrate when they come into contact with saliva, to enable swallowing without chewing or the need for water. FDA guidelines state that ODTs should disperse in less than 30 seconds. Zydis ODT is made using a lyophilization process that results in extremely rapid disintegration – as fast as three seconds, once the dosage form is placed on the tongue. The drug is presented in a finely dispersed form for rapid onset, and depending on its specific properties, may be absorbed from the oral cavity and avoid first pass metabolism in the liver.
“Zydis ODT was the first orally disintegrating tablet. Before that, there were chewable tablets, capsules, and lozenges, but no solid dose that disintegrated instantaneously in saliva, with minimal patient input. The only alternative at the time was a suspension or liquid product, but these can be very inconvenient to take and come with concerns around the accuracy of dosing,” explains Ralph Gosden, Head of Product Development at Catalent.
“After the launch of Zydis ODT, other products began to emerge to try and match the performance using different approaches, such as compressed tablets incorporating high levels of disintegrating agents,” says Rob Smith, Vice President, Product Development and General Manager for Catalent’s early development site in Nottingham, UK. “You can create an ODT in a variety of different ways, but some of the tablets produced had very slow disintegration times, which is why the FDA deemed it necessary to issue guidelines around ODTs in 2008, to clarify the expectations of ODTs. In some products, disintegration time was over a minute.”

As well as a disintegration time of less than 30 seconds, the FDA guidance encourages manufacturers to also consider tablet size and weight; an ODT that is too large or that disintegrates too slowly could be a choking hazard. The guidance states (1), “While tablet size or weight is not explicitly included in the definition, you should consider the effect large tablets have on patient safety and compliance. We generally recommend that the weight of the tablet not exceed 500 mg; however, if a tablet intended for use as an ODT weighs more than 500 mg, its ability to perform effectively as an ODT should be justified based on product performance. For such products, the extent of component solubility (e.g. tablet residue, need for liquids) can influence the acceptability of the product being labelled as an ODT.”
Making the Magic Happen
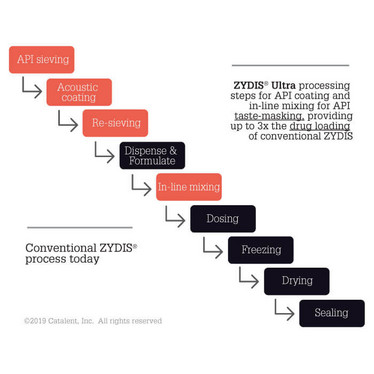
A Zydis tablet is made using lyophilization. Firstly, the bulk API is formulated into a liquid solution or suspension. The liquid is then precisely filled into pre-formed blisters and passed through a cryogenic freezing process to control the size of the ice crystals, before the units are transferred to large-scale lyophilizers.
Given that tablets made by freeze-drying can be sensitive to environmental conditions, the sealing and packaging process is also important. The blisters are subject to a heat-seal process to protect the product from varying environmental conditions and to ensure long-term stability.
Zydis is also capable of delivering some proteins and peptides orally (Zydis Bio), as the drug has the potential to bypass the GI tract. The lyophilization based manufacturing process and its low temperatures reduces the potential for heat damage to the biologics, but the process requires careful formulation with the right selection of ingredients to optimize in-process stability.
Patients in mind
Oral drug delivery is well-recognized as a convenient, economical and safe route of administration. And much has been written about the specific advantages of ODTs (2,3). From a patient perspective, ODTs offer the convenience of medication “on the go” and without water – which is particularly useful for certain medical conditions, such as a migraine which can hit unexpectedly, and where there is not access to water. And if a patient feels nauseous, as with motion sickness, consumption of any liquid may be best avoided. Of course, there are also people who just do not like swallowing tablets at all.
“Sometimes the barrier is psychological, but in other cases the ability to swallow may be severely hindered by age or a medical condition – consider an elderly patient with dysphagia, for example, and the fact that many geriatric patients are taking multiple tablets per day,” says Smith. “Swallowing tablets or liquid suspensions can also be an issue for very young pediatric patients and there are particular concerns about the dosing when children spit out their medicines.” Studies have shown that ODTs are particularly popular for pediatric patients, with many medical practitioners believing that liquids (the most common type of dosage form for children) could be replaced by ODTs (4).

But there was also another driver for the development of the Zydis ODT technology: the continuing need for improved patient compliance. Poor compliance has been reported as one of the most common causes of nonresponse to medication. In some cases, the convenience of the dosage form could help encourage patients to take their medicine at the right time, but there are also patients who refuse to take medicine at all.
“This is a common issue with psychiatric conditions, such as schizophrenia, depression, or bipolar disorder,” says Smith. “Some patients require supervised administration to prevent them from regurgitating the medicine or hiding it – concealing a tablet in a cheek to eventually spit out is a common tactic adopted by some patients to avoid consumption. Once Zydis ODT is in the mouth, you can’t spit it out because it disperses so quickly. Because of these advantages, it has been used for a range of medicines targeting psychiatric disorders and can even be employed for medicines designed to help heroin addicts.”
Studies have also shown that nonadherent patients with schizophrenia or bipolar disorder treated with orodispersible formulations are less likely to be hospitalized or suffer relapse compared with those patients taking standard oral coated tablets (5). Other studies have shown that ODTs can improve patient compliance (6). “We’ve also conducted our own research and have found that ODTs can improve patient compliance – 98.5 percent compliance versus 81 percent compliance with a standard oral treatment,” says Susan Banbury, Head of Zydis Formulation at Catalent (7).
From a business perspective, the team also believe that such unique dosage forms are harder – if not impossible – to counterfeit.
Ultra new tricks
The first Zydis ODT to receive approval from the FDA was Claritin (loratadine) in December 1996, but today more than 30 products have been launched in 60 countries, including both prescription and over-the-counter medicines. Catalent manufactures over one billion Zydis ODTs every year in 17 different therapeutic areas. But if the first Zydis ODT was approved in the 1990s, then how did the latest iteration win The Medicine Maker Innovation Awards over 20 years later?
Although offering many advantages over other solid dosage forms, the original Zydis technology had some limitations with drug loading and taste-masking – with the latter being particularly important for a product dispersing in the mouth. And so, Catalent’s research team worked on the next generation, called Zydis Ultra technology.
“There were taste-masking options for the conventional Zydis formulations, such as flavors and sweeteners, or by combining with an ion exchange resin or cyclodextrin. For some APIs, these strategies work well, but in other cases the effect was limited, or could not accommodate the required tablet dose. For example, in general, the more soluble the API, the higher the risk that the taste will be unacceptable,” says Banbury. “Coated APIs for taste masking purposes have been available for some time. However, incorporating them into the conventional Zydis formulation using standard processing methods, while maintaining the integrity of the coat, was challenging. Zydis Ultra technology allows a coated API to be used. And drug loading is three to four times higher than with a conventional Zydis ODT.”

During the formulation of any solid oral dosage form, the mixing process is crucial, with a potential impact on compression, dissolution and other finished product characteristics. Mixing can also be affected by conditions such as device capacity and product mass, so you need to choose the right technology to get the right outcome. The secret ingredient in Zydis Ultra ODT? It’s a mixing process, which has been in development since around 2012 and uses resonant acoustic mixing technology to coat the API. “The technology was initially developed for the ammunition and ballistics industry, where it received a lot of US federal investment. Zydis Ultra technology was made possible because of it,” says Craig Scott, Director of Product Development at Catalent. “Resonant acoustic mixing works by controlling vibration through acceleration and frequency. The materials being mixed do not come into contact with any moving parts, with the exception of the vessel, so it’s considered a safe process that generates no static.”
Acoustic mixing can be used for dry mixing materials with various flow aids, but for Zydis Ultra ODT, the technology is used to form a continuous polymer layer around API particles, without solvents. “The easiest way to describe? It’s like the materials are being forcibly shaken,” explains Scott. “API particles are mixed with micronized polymer aggregates using an acoustic vibrator at a very high velocity. This creates a significant amount of energy (up to 100 times the force of gravity), which causes acceleration and collision of particles, and raises the temperature until the dry polymer becomes malleable and molds itself around the API in a very thin layer. No solvents are involved and the API retains 70 to 85 percent of its potency.”
Resonant acoustic mixing can coat particles less than 100 μm with minimum impact on final particle size, whereas conventional coating typically results in significantly larger particles that can lead to a grittier mouthfeel in ODTs. Researchers at Catalent have been able to formulate both ibuprofen and acetaminophen using the new technology (3).
The “Wow” Effect
“I was working for a competitor to Catalent when I was given a placebo of Zydis. I tried it and was so wowed by the technology that I decided I wanted to work for them. I quit my job, joined Catalent, and I haven’t looked back! It’s been rewarding to see the technology go from strength to strength, with Zydis Bio and now Zydis Ultra. In the future, I hope we can use the Zydis technology to deliver vaccines. The coating doesn’t allow for the sublingual uptake that you would normally want with a vaccine, but we have a few tricks up our sleeve that I won’t give away! I think Zydis has been a major breakthrough, shaking up the traditional platforms for oral solid dosage. Zydis Ultra has so much potential with the extra dose loading and I think it will fulfil more promises beyond what we’ve seen with Zydis.” – Rob Smith.
“Working with a technology that genuinely meets the needs of specific patient groups has been a real pleasure. The needs of demographics who are often excluded (including pediatric and geriatric populations) are addressed in ways that simply cannot be achieved using conventional approaches. Zydis definitely has a “wow” effect when you first try it. I’m looking forward to getting this dosage form to more patients and indications with the release of Zydis Ultra.” – Susan Banbury.
“It’s a real privilege to work on a dosage form that you can really believe in. I’ve been working on Zydis for about 15 years and seeing it evolve over the years has been great. I still remember the feeling when I saw Zydis Ultra running down the commercial lines without any significant issues. It was great to know that we could really create this on a commercial scale! On a personal note, I have a young family. Administering medicine to children is difficult, especially when they spit out liquids, which ruins the dosing! When you give a young child an ODT, you know you have given them an accurate dose. I find that very comforting – both as a parent and as a developer. It also gives a positive medication experience to children.” – Craig Scott.
“We then formulate the coated API into a typical Zydis formulation so that it maintains its fast disintegration time. It took a lot of work to adapt and refine the approach from the ammunition industry and then scale it up to something that we could commercialize!” says Scott. “There’s a huge amount of work involved when looking at the various different options – and one of our key goals was to ensure that the new formulation could be filled on our current existing lines. Our engineering and operations teams have had to work very closely together. The dosing, in particular, was a very big challenge.”
For some extra help in adapting the technology, the company also approached the New Jersey Institute of Technology. The challenge was achieving a complete, perfect coating of the API particles so that there is negligible release of the API for the first 90 seconds, but that the material is then fully available for gastrointestinal absorption. “The team also had to address how we integrate a very delicately coated API particle into a liquid solution or suspension without damaging the coating while ensuring that the taste-masking characteristics are successfully maintained,” says Smith. “We have some patents on this under submission, so in the future we’ll be able to talk more freely about how we take these delicate API particles through the manufacturing process.”

And coming up next…
According to the Catalent team, there has been considerable interest in Zydis Ultra, particularly for products that have known taste-masking issues. “We have four products in the pipeline at the moment. The first is planned for launch around 2021; we’re ramping up to supply the bioequivalence studies at the moment,” says Scott. “Clinical data is due back later this year, and we have also been conducting consumer preference studies. Our previous consumer preference studies with major pharma companies using a Zydis Ultra placebo garnered outstanding feedback. I like to think the outlook is positive!”
And because of this outlook, Catalent announced a $27 million investment in March 2019 to help commercialize Zydis Ultra technology, given that a number of development programs are now reaching the full development stage. The Zydis development and manufacturing operation is located at the company’s 250,000 square foot site in Swindon, UK.
Even with the additional opportunities available with Zydis Ultra, the original Zydis technology is here to stay, says Scott. “For some APIs, the original Zydis technology remains the most appropriate option, but Zydis Ultra builds on this expertise and in combination with the technology continues to go from strength to strength; I’ve been involved with Zydis for 15 years and this is the biggest development pipeline I’ve ever seen.”
Innovation Awards 2019
Do you want to share the story behind your technology in a future issue of The Medicine Maker?
In our December 2019 issue, The Medicine Maker will showcase the top 15 technologies to have been released throughout 2019. The final winner will be decided by a public vote and – just like Catalent – will be able to tell the story behind their innovation in a 2020 issue of The Medicine Maker.
The nomination form for the 2019 Innovation Awards is now live: tmm.txp.to/innovations19-noms
The rules?
The technology must have been released (or planned for release) in 2019 and it must be expected to have a significant impact on drug development or manufacture.
The innovation can be a piece of equipment, IT software, formulation technology, drug delivery method or any other innovation that you think could fit the bill.
Questions?
Contact the editor: [email protected]
- FDA, Guidance for Industry, “Orally Disintegrating Tablets” (2008). Available at bit.ly/2WjnANp. Last accessed May 21, 2019.
- FB Abay, T Ugurlu, “Orally Disintegrating Tablets: A Short Review,” Journal of Pharmaceutics & Drug Development, 3 (2015).
- R McLaughlin, L Grother, M Bayru, “Orally Disintegrating Tablets: A Dosage Form Designed for Difficult Patient Populations,” American Pharmaceutical Review (2016).
- H Alyami et al., “Current opinions and recommendations of paediatric healthcare professionals – The importance of tablets: Emerging orally disintegrating versus traditional tablets,” PLoS One, 13 (2018).
- D Novick et al., “Comparison of clinical outcomes with orodispersible versus standard oral olanzapine tablets in nonadherent patients with schizophrenia or bipolar disorder,” 11, 1019-1025 (2017).
- C Cecchi, PL Canonico, “Pharmaceutical formulations and adherence to pharmacological treatment in psychiatry: the example of oral disintegrating tablet of olanzapine,” Riv. Psichiatr., 37, 30-39 (2012).
- S Hamlen, K MacGregor, “New data shows drug delivery has positive impact on patient compliance,” Drug Development & Delivery, 11, 7, (2011).

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















