Keep Calm and Use Pools
How pooled cell populations helped accelerate COVID-19 vaccine development – and what that means for the therapeutics of the future
Trent Munro | | Longer Read
sponsored by Lonza
The COVID-19 pandemic gave a huge boost to the vaccine field, with scientists throwing every technology they had into the arena to help find a vaccine candidate as quickly as possible. My group contributed to the fight by putting our “molecular clamp” technology platform forward.
The surface proteins on many viruses, including SARS-CoV-2, are highly complex molecules that can be unstable when made in isolation. One challenge is the fact that these molecules exist in two main conformations, depending on pre- or posttarget binding (pre-fusion conformation or post-fusion conformation). When viral surface molecules are made in isolation, they enter into an energetically favourable form: the post-fusion form, which obscures important epitopes that may be important for a vaccine to ensure right protective immune response. To overcome this challenge, you can synthesize the molecule in a way that “locks” it into the pre-fusion form, which is most recognized by the immune system and more able to generate the right type of immune response.
For SARS-CoV-2, it is possible to design a “lock and key” mechanism by placing new amino acid substitutions into the molecule. The approach builds on previous work with closely related viral proteins from MERS and SARS, and is used for many COVID-19 vaccines; however, it is also very specific to this type of spike protein and is not a broadly applicable technology. Moreover, it requires a significant structural understanding of the molecule.
At the University of Queensland, Australia, we’ve been working on a different approach to vaccines that we hope could become a platform technology applicable to a range of different viral proteins; notably, we were working on this long before COVID-19. In short, we genetically fuse the trimer forming domain to viral surface proteins, which locks the molecule into its pre-fusion conformation. We refer to this as a molecular clamp; after all, it essentially clamps the trimer in place. We’ve shown that the approach works for a range of different viral proteins, including Ebola, influenza, RSV, and MERS. We have also managed to attract from The Coalition for Epidemic Preparedness Innovations (CEPI).

In early 2020, we were preparing for a clinical trial of an influenza candidate, which would be the first human proof-of-concept for our molecular clamp approach. We used CHO expression technology to make the vaccine, which worked, but based on my experience from industry, I knew there were better systems out there that could drive high levels of expression. I was fortunate to be aware of the Lonza GS expression system and the power of genetically engineered GS knockout CHO cells.
When we pivoted to COVID-19, we had to think about technologies that would allow us to produce enough doses of the vaccine (if successful) to vaccinate millions of people. I reached out to Lonza because I felt their GS XCEED expression system was the most powerful and easily accessible CHO expression platform.
It starts with understanding
Today, we understand how different expression systems work, the importance of critical quality attributes for the molecules we make, and how to control them. We also have access to different types of bioprocess controls, as well as chemically defined media. However, the regulations were set many years ago and have not adapted to new technologies. We also need to consider that there have been examples where companies have made poor decisions during cell line process development, so regulators are keen to ensure no shortcuts are taken that might impact product safety or efficacy.
When making a cell line to produce a therapeutic protein or vaccine, there is a perception that cloning from a single cell creates a higher degree of overall stability, and creates a robust cell line for manufacturing.
What Is a Pool?
By Alison Porter, Head of Expression System Sciences at Lonza
Simply put, a pool is a mixed population of stably transfected cells.
During transient transfection, the recombinant DNA does not integrate into the host genome and therefore does not replicate, so it will eventually be lost as cells divide. On the other hand, stable transfection begins transiently, but is followed by an infrequent but critical event where recombinant DNA will integrate into the host genome, meaning the product gene can be replicated. Descendants of those transfected cells will also express the product.
Transient transfection is typically used during the drug discovery and screening stage when researchers are assessing multiple variants and need relatively small amounts of material fast. Stable transfections are then used when creating a suitable cell line for manufacturing. This is because integration is important if longterm gene expression and large amounts of material are required. Typically, stable transfection will also require a cloning step to meet regulatory requirements.
A pool can be described as a halfway house between a transient transfection and a clonal cell line from a stable transfection – in fact, a pool is a typical starting point from which to clone. A pool takes longer to create and obtain material from than a transient transfection, but less time than a clonal cell line. However, although a pool can generate more material than a transient cell line, it can generate less than a clonal cell line (but this difference is reducing as the industry becomes more familiar with pools and as technologies and techniques advance).
At Lonza, we first started investigating and using pools in the early 2000s. We initially used pools to supply some customers with material very early on in development, but we quickly found that pools became a main part of the cell line construction workflow because we found that they could help to significantly reduce timelines. Today, the use of pools has increased substantially; they are frequently used to select the best starting point to clone from, to get a head start on upstream process development, and to develop material to feed other project stages.

Over the last 10 years, there have been many interesting conversations about the use of pools in the industry, including whether it’s possible to use material generated from pools for toxicology studies or even for phase I trials. But the real accelerant that has pushed pools to the front and centre is the COVID-19 pandemic – where there was a need for significant reductions in timelines. I expect us to see the use of pools being pushed even further in the future, particularly for developing antibodies, where the industry has so much experience already.
Lonza’s GS GENE EXPRESSION SYSTEM is well established and can help create transient material, clonal cell lines, and pools – and we provide the protocols as well as actual physical materials. We use the system ourselves when creating cell lines and developing processes for customers, but we also out-license the same system for customers to use in their own laboratories.
Trent Munro and I have known one another for some time; he was aware of our technologies and contacted us to request access to the GS SYSTEM in his lab – in short, that’s how our fantastic collaboration began!
Regulators need to ensure that manufacturers will make a safe, effective product the same way with each manufacturing run, and many would argue that using a clonally-derived cell line is the golden path.
I would argue that this is not necessarily the case. The most important element is knowledge; you must understand your expression system and how your expression system matches your bioprocess, and you must understand how to isolate a population of cells to create a robust manufacturing platform. This connectivity and understanding – and being able to back it up with an appropriate data package – is far more important than whether you are using a clone, a pool, or a different origin for the manufacturing process. With pools, you simply need the characterization and comparability studies to demonstrate to regulators that the material you are generating is suitable for purpose. Ultimately, regulators are data driven and will make decisions based on the quality and robustness of your data package.
Harnessing the full potential of pools
I believe that the combination of stable pools and well-characterized processes is a powerful method to accelerate early development. And new technologies, such as the Lonza GS platform, are amenable to the creation of stable pools that are suitable for manufacturing. These pools can potentially be used for early-stage material, early clinical, or even for commercial manufacturing, depending on the data for the individual program and molecule.
In our vaccine program, we had no time to use clones. COVID-19 forced us to move fast. We worked with partners to quickly define our critical quality attributes and set the bar for comparability – and that gave us the confidence to use a pool-based expression system.
We demonstrated biological proof of concept by looking at animal studies in mice, hamsters, rabbits, and ferrets – at the time we didn’t know what would be the best animal challenge model for COVID-19. It was critical that we generated high quality material to go into those studies and for our preclinical GLP toxicology studies. The only way we could generate this material fast enough was to use pool-derived material. That said, we did also produce some clone material – and it was indistinguishable from what we produced from pools.
We were fortunate to be able to work on our program at different locations in Australia and run parallel clinical lots. The Lonza production process worked well and we tech transferred to a larger biotech company – CSL – which was able to transition to commercial, clonally-derived production in just a few months. This speaks to how well the CMC process was set and how well the pool process worked. The other piece of the puzzle was the technical support from the Lonza team. This made for a truly seamless tech transfer experience from construct generation, through to pool creation, and then upstream process optimization. Their deep understanding of the system allowed for first time success of our clinical lot. Despite Lonza being busy with other manufacturing projects they were always responded quickly to any queries we had and were keen to help us as much as they could.
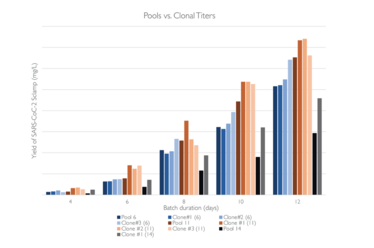
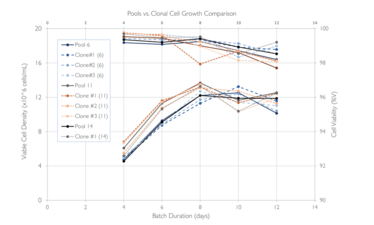
Abridge fed batch culture expressing SARS-CoC-2 Sclamp (~600kDa) in shake flasks using the Lonza GS XCEED system with v8.10 processes. Solid lines represents pools, dotted lines are the clonal cell lines, number in the bracket for clonal cell line shows which pool they were derived from. Cell growth and titers produced by pools is comparable to those of the clonal cell lines.
Reviewing the results
Our program went through phase I and delivered good immunology data in terms of the level of immune response. But we hit an unusual snag. In our molecular clamp approach, the trimerization domain contained two peptides derived from the GP41 HIV protein – and the GP41 molecule is used in a number of screening tests for HIV. Participants who received the vaccine not only generated an immune response against the COVID-19 spike protein but also to small elements derived from GP41, which cross-reacted with some HIV screening tests to produce a false positive. At the same time, efficacy data began to be released for the Oxford-AstraZeneca vaccine, as well as the Pfizer and Moderna programs.
We had to decide whether to pause development or push forward into phase III with the caveat that the vaccine could cause a false positive in some HIV screening assays. When we discussed the issue with HIV experts, everyone agreed that diagnostics can be changed, so it was a tractable issue. However, the reality of interfering with the HIV diagnostic algorithm is complex. In some countries, for example, a positive result in a lab can lead to the patient being given antivirals without a secondary check. Clearly, inducing a false positive for HIV with our vaccine had the potential to cause issues, so we ultimately decided to halt our COVID-19 program.
I’ll be honest; it was a tough decision to make! We were confident it was a good vaccine and, as it was made in a CHO cell system, it only came with mild side effects. If we had proceeded with the program, I think we probably would have been the first protein-based COVID-19 vaccine to reach the market. Right now, there’s still no protein-based vaccine widely approved for COVID-19, but we are hoping that changes any day…
On the positive side, however, we do now have human validation that our molecular clamp approach can drive a robust immune response. Over the past several months, our team has been busily exploring a new trimerization domain that is not derived from GP41. We are calling it “Clamp 2.0” and we’re looking at a range of different viral antigens and potential vaccines for commercial targets. We’re also keeping an eye on the COVID-19 variants and we continue to work with CEPI.
Accelerating beyond the pandemic
Today’s sophisticated analytical tools allow us to understand molecules very early on; we can pull apart a molecule and really understand what residues are important at an amino acid level, what affects activity, what could change, what is a liability, and so on. It doesn’t matter if materials are generated from a pool or a clone – as long as they are characterized accurately. I believe that pools of genetic material could become a regular tool for speeding up the development process. Getting into phase I studies is absolutely critical and every day you can save is beneficial! Having an artificial separation between clonally derived populations or pools seems like an antiquated way of working. We really should just be focusing on the quality of the molecule and how we use that material and our ability to create a reproducible process.
We’re really pleased with our partnership with Lonza. Being based in Australia, we are a long way from Europe and the US, and having access to the Lonza system during the pandemic really empowered our work. We’re now working on several different COVID-vaccines with researchers in Australia, and we’re also looking at the production of monoclonal antibodies for COVID-19. We wouldn’t be able to do that with the quality and the speed that we have today if it wasn’t for the generosity and the partnership with Lonza. Lonza has spent years developing the GS SYSTEM to get it to where it is today, and the combination of the GS knockout cell line and cell culture platform result in a robust upstream platform. The GS XCEED expression system brought great benefits to our project because it allowed us to move fast – speed was clearly crucial at the start of a public health crisis.
But this technology is not just applicable to a pandemic. Getting new therapeutics to patients as quickly as possible – in all therapeutic areas – is important. And technology like this can help developers to move much faster. I firmly believe that using pools of genetic material early in development can bring huge benefits to a project.
pharma.lonza.com/experts-in-expression
Why Did We Use a CHO Expression System for Vaccine Production?
With Trent Munro
Some companies are using a baculovirus approach to a COVID-19 vaccine, including Sanofi and Novavax. These vaccines have very different types of post-translation and modification profiles compared with a vaccine derived from mammalian cells. So far, the immunology data coming out of these vaccines looks good, although we’ll learn more as additional clinical data emerges or as they are rolled out more broadly
In our program, we wanted to replicate the glycosylation pattern of the virus as closely as possible – CHO makes sense here. But we also wanted a production system that was a good fit for the installed base of bioprocess capabilities available in CDMO networks and across the biopharma industry – and capacity for CHO production technology is huge. Finally, CHO secretes molecules at a good yield.
Clover Biopharmaceuticals is another COVID-19 vaccine developer that is using a CHO-based production system – and promising data has come out of their phase III program. For me, Clover’s data proves the validity of using CHO as a production system for COVID-19 vaccines. And, more broadly speaking, I believe CHO is a sensible choice for vaccine production.
Senior Group Leader at the Australian Institute for Bioengineering and Nanotechnology at the University of Queensland, and Director of the NCRIS funded National Biologics Facility, Australia.



















