Keeping Fit With Age
The saying goes that with age comes wisdom, but in manufacturing it can also bring about unreliable facilities and equipment. Proactive stress testing is a must to keep facilities fit and healthy.
sponsored by CRB
Like everything else, sterile manufacturing facilities age and performance deteriorates over time. Many of the first and second generation biomanufacturing facilities are now 15-20 years old, so it’s no surprise that sterility issues and product recalls are on the rise. One of the early warning signs that a facility is in need of some attention is an increase in batch failure rates – companies want batch failure to be less than 1 percent, but the Parenteral Drug Association has noted that it can be much higher: “Alarmingly, 80 percent of respondents to a survey conducted by the Parenteral Drug Association in 2016 had batch rejection rates of up to 4 percent due to a lack of sterility assurance or the potential thereof, and 15 percent of those respondents had rejection rates of 5-10 percent” (1).
Issues with facilities and equipment can lie dormant as operators or maintenance technicians make small tweaks to compensate, but in time this can lead to other problems. Increasing contamination rates in older bioreactors are one common problem, for example, and the kneejerk reaction is often to turn up the steam cycle or temperature, but this can lead to gasket or other component failures. If equipment is becoming a source of contamination or is difficult to clean, then it is likely to be outmoded and you may need to consider replacement. Also, bear in mind that older equipment designs tend not to be the most efficient anyway – they may have nooks and crannies that complicate cleaning, or may require significant manual intervention, so it’s good practice to continuously evaluate newer systems.
Sometimes it is not necessarily the age of a facility or its equipment that are a problem, but simply the fact that times and product demands have changed, and that existing equipment can’t keep up. Problems in facilities of any age can also stem from lack of operator training or failure to follow standard operating procedures. If procedures and training are not adequately documented, issues can arise as companies lose legacy employees and bring in new operators or technicians – who may be eager but unwittingly undo procedures. I am sure that everyone reading this is also aware that it is all too common for one step to be skipped because it is a little troublesome or because people don’t think it matters… They might get away with it the first time, but then it becomes habit – and in time, other shortcuts or small problems will culminate to form a much larger issue.
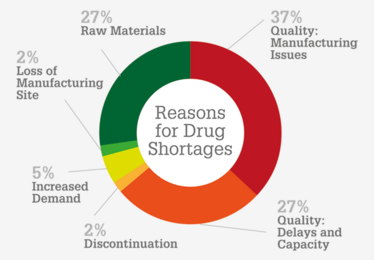
Reasons for drug shortages. Data from the FDA (https://bit.ly/2EqEX1I). Quality manufacturing issues and quality issues related to delays and capacity are the biggest contributors to drug shortages.
Fit facilities
When addressing a problem, you should never try to fix it in a vacuum; it must be properly socialized and vetted among all of the applicable departments, such as operations and process staff, quality assurance, validation, maintenance, quality control etc – so that you can ensure the solution doesn’t cause other issues down the line. However, it can be very difficult to identify what the root cause of the problem – and thus the best solution – actually is. In my experience, people are very quick to assume they know what has caused the problem, but I always recommend a thorough, unbiased root cause analysis. When contamination arises, various groups within an organization will often each have their own view of the source, leading to disagreements and months of wasted time in chasing down assumptions that ultimately turn out to be incorrect. Often, a root cause is not immediately discoverable until you have performed an impartial, passion-free analysis.
I recommend using a full “stress test” to identify problems and design issues that may require remedial action. This should examine all operations and determine where the soft points may be – and whether the facility is still fit for purpose. A stress test is a comprehensive analysis that should begin with discussions and interviews with all of the different groups involved in maintaining the facility. You should examine manufacturing processes, equipment, training and history, and be on the lookout for any deviations. Also look at batch records, change control and corrective action plans for problems that may have arisen – in case they have had unexpected effects. With engineering and validation, you can get a sense of how manufacturers have been tracking equipment performance by looking at equipment alarms that have come up recently. And from there you follow the threads back… it is quite common for a problem to start with an alarm that has been ignored, or thought to be irrelevant. You also need to talk with maintenance groups and look at work orders, unscheduled maintenance, and maintenance frequency. Is it strictly time-based, or are they looking at how hard or how frequently equipment is run? In some facilities, equipment is run close to 100 percent capacity – and in that case preventative maintenance and changes may need to be made more frequently to avoid problems.
Plan and prepare
It could be that the stress test reveals no problems, which is fantastic news! But it doesn’t mean you should be complacent – you still need to plan for timely repairs and replacements to ensure that your facility continues to be fit for purpose. If nothing requires immediate action then you can use the time for future planning. For instance, if you have a piece of equipment that can only perform 50 batches a year, but you’re projecting the need for 75 batches in the next two or three years then you need to start planning how you will adjust. Also, don’t forget that your staff will need ongoing training as new technology is integrated and as quality standards change.
Every now and then I come across someone who says, “Why don’t we just let things fail and then fix them? What are the consequences of just waiting until something breaks?” My biggest advice is to always be proactive with your facility before issues arise. Once you have a problem, your capacity plan is at risk. Stress testing a facility proactively allows remediation or upgrades to be planned, meaning you can choose the best time to shut down a facility for any work and fit capital spending into budgets. And remember, the FDA expects a facility and its equipment to be designed and maintained for what you intend to produce in that facility – it is cGMP (current good manufacturing practice) for a reason. The FDA emphasizes the word “current” and grandfathering in an old approach or an old piece of equipment is no longer considered acceptable. Your technology does not have to be leading edge, but it must be accepted and you must have data to back up your approach.
In the pharma industry, all manufacturers have a duty to ensure that their facilities are up to scratch. The key goal is to supply patients – contaminations and drug shortages prevent medicines from reaching patients and can cost lives. This is something we have a duty to avoid. There is nothing worse than having a need in the market for a medicine you produce – and being unable to supply it.
Allan Bream is Associate, Lead Biopharmaceutical Specialist, at CRB Raleigh, North Carolina, USA.
- PDA Aseptic Processing Survey 2016



















