mRNA IVT: A Modern Balancing Act
Optimizing mRNA in vitro transcription (IVT) processes and ensuring representative materials ahead of scale-up is important – high-quality kits are a great starting point
Jeneffer England | | 6 min read | Technology
sponsored by Thermo Fisher Scientific
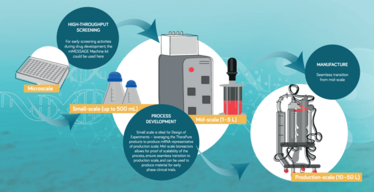
As the pandemic turns endemic, it is clear that messenger RNA (mRNA) vaccines are also here to stay. The ease with which we can modify mRNA sequences provides a truly flexible platform that can target a wide variety of diseases. Unsurprisingly, the market is expanding rapidly; more and more companies, including traditional viral vaccine manufacturers, are investing in mRNA vaccine technologies. The rise of mRNA can be described as a genuine paradigm shift—one that has inevitably led to a new focus on the methodologies and practices that allow for successful scaling and manufacture of mRNA products using in vitro transcription (IVT).
But mRNA vaccines are relatively new, and though there are many more players in the field, they are not all on equal footing. Throughout the pandemic, I have been working on mRNA IVT scale-up; here, I would like to present some of the lessons I have learned on my journey, starting at the microliter scale. For those considering a move to mRNA technology or in the midst of scale-up, I hope to shed some light on the key challenges you may face, and some solutions to help you on your journey.
Simple—but not without challenges
Making an mRNA therapeutic is relatively straightforward; for one thing, there is no need to rely on living cells to express a protein or antibody of interest, which removes a headache. While the industry has certainly learned to live with this headache—in fact, developers have optimized processes well beyond early expectations—removing it can certainly create opportunities for efficiency and innovation. To manufacture mRNA we rely on a biochemical reaction between an enzyme, a DNA template, and nucleotide triphosphates (NTPs).
If you’re new to mRNA, you will almost certainly start at microscale, where typical volumes for standard reactions are below 1 mL. My tip here is to use a high-quality transcription kit (for example, the Invitrogen™ mMESSAGE mMACHINE™ Transcription Kit) to maximize the yield of capped mRNA. Remember that kits are the product of a great deal of expertise—so make use of that expertise.
After the appropriate incubation (2-4 hours at 37°C), the mRNA must be purified (in our IVT scale-up project, my team used the Invitrogen™ MEGAclear™ Transcription Clean-Up Kit) and quantified using a variety of analytical techniques.
When it comes to optimizing the IVT process, we have a number of challenges to address. One challenge is minimizing the formation of double-stranded RNA (dsRNA), which is not removed by the affinity resin and requires an additional polishing step. Research is ongoing into strategies to reduce dsRNA, either by modifying the enzyme used in the reaction or by optimizing the template sequence itself. Unfortunately, current techniques employed to assess the presence of dsRNA (for example, dot blot assays) tend to be tedious, time-consuming, and non-quantitative. Indeed, analytical techniques to fully and efficiently characterize mRNA products are works in progress—a testament to how new mRNA therapeutics really are.
As IVT volumes increase to around 10– 20 mL, larger quantities of bulk reagents are required (we use the TheraPureTM enzymes and NTPs—but lessons learned at the microliter scale can be applied). Nonetheless, with higher volumes, the demands of purification increase, which necessitates different equipment; here, oligo dT columns come into play (for example, Thermo Scientific™ POROS™ GoPure™ Oligo (dT)25 Pre-packed Columns). Oligo dT resins selectively capture the mRNA by its poly(A) tail, allowing any truncated species as well as excess enzyme, NTPs, and template DNA to flow through. Your freshly synthesized mRNA can then be eluted with TE buffer or water.
Balance and scale
When i t c omes t o s cale-up, m y fi rst tip is to ensure there is sufficient plasmid template or PCR product for the desired output—and that may not be as straightforward as you think, especially as mRNA length increases. The concentrations of reagents also affect yield; for instance, the concentration of NTPs can be optimized for greater yields —and what works well at the microliter scale may not be sufficient at the small scale or production scale. This is equally true for the concentration of the RNA polymerase enzyme; certainly, I have found that higher concentrations of T7 RNA Polymerase increase yield, but given the cost of enzymes, a balance must be struck.
Reaction time is another balancing act. Longer reaction times can decrease the level of truncated species, but can potentially increase levels of unwanted by-products, specifically dsRNA (another reason why scientists are busy working on solutions to address dsRNA directly).
Finally, it is important to note that the balance is much more likely to be tipped in your favor with high-quality starting materials and raw materials. Moreover, the output—in terms of maximizing yield and minimizing by-products—at a given scale is likely to be more representative of the next jump in scale with higher-quality starting materials and raw materials. And that is particularly important when it comes to understanding purification needs. For example, larger quantities of dsRNA, if left unresolved, may necessitate an extra polishing step (using hydrophobic interaction chromatography or ion chromatography). In short, whatever inputs you use in your design of experiments (DOE) and any process optimization are really important when it comes to scaling up. Some aspects will necessarily change, but the greater the level of predictability, the smoother the scale-up process—and that is especially key given the valuable nature of the starting materials and raw materials.
Keeping pace with a moving target
Spurred on by the recent success of mRNA vaccines, many companies are taking their first steps into this new and exciting world. Gaining a thorough understanding of reaction variables and finding a robust way to scale mRNA products will help deliver a great reward for successful companies: a highly valuable platform that allows rapid acceleration of future therapeutic candidates. For companies at the beginning of their mRNA journey, high-quality kits offer an excellent starting point, allowing rapid, reliable process development and providing insight into potential challenges during scale-up.
The future of mRNA manufacturing will almost certainly demand new and improved downstream processes and analytical techniques, and Thermo Fisher Scientific is committed to making great strides in both areas. We already have expertise and solutions in place for the current wave of mRNA therapeutics, and we continue to explore new resins that enable faster mRNA product processing with the same yield and recovery, as well as more efficient and reproducible analytical techniques and methods. Meanwhile, we are also working hard on solutions to support the next wave of mRNA products—self-amplifying mRNAs and circular mRNAs, for example—which will doubtless bring new challenges.
Given the rapid pace of advancement in the field of mRNA therapeutics, it is hard to imagine the landscape five years from now—let alone farther into the future—but I have a strong feeling that many lives will have been touched by this marvel of modern medicine.
Jeneffer England—mRNA Vaccines & Therapeutics at Thermo Fisher Scientific



















