Painting a Better Picture of Quality
When seeking to measure quality across an organization, what is the right approach? We propose to use the synergies of Quality and Operational Excellence programs – and we are sure you will see the benefits.
The FDA’s approach to quality oversight has evolved in recent years, particularly with the establishment of the new Office of Pharmaceutical Quality (OPQ). OPQ exists within the Center for Drug Evaluations and Research (CDER) as a single unit dedicated to product quality with a simple mission: “to assure that quality medicines are available for the American public”. In 2013, the FDA announced in the publication of the FDA Administration Safety and Innovation Act (FDASIA) that they intended to examine the use of selected quality metrics (the exact metrics will be decided upon and collected during an FDA quality metrics program) to support their risk-based inspection program. And more recently, the FDA published its draft guidance for industry related to the quality metrics request (1).
But to paint the entire picture of a site and an organization in terms of quality performance, we must go beyond a limited set of metrics that relate to quality only in a narrow sense. In our view, a more holistic approach to quality must be integrated both into the operation of the manufacturing facility and its underlying quality culture. Fortunately, the tools already exist. Indeed, over the past 10 to 15 years, the pharmaceutical industry has become increasingly aware of formal Operational Excellence (OPEX) programs, where the initial driving forces behind implementation were improvements in operational efficiency – and the associated potential cost savings. But recent developments in more mature OPEX programs have shown that there are other considerable benefits, such as the reduction of variation. The stability of quality management systems of companies with a more mature OPEX program is significantly higher compared to their competitors. OPEX programs help companies to increase their equipment and process stability. Notably, commensurate improvements in quality have also been gained using OPEX programs through measurable stabilization of the organizational systems responsible for equipment and facilities, quality management, inventory control and management oversight.
Based on our experience and in-depth research of the St. Gallen OPEX research team that correlated quality outcomes across the supply chain with available OPEX performance data at a given site (2), we developed a framework to assess:
- Supplier reliability: the service level supplier and the complaint rate related to supply issues.
- Production stability: production related indicators, such as overall equipment effectiveness, unplanned maintenance and right first time, in combination with quality-related measures like rejected batches, scrap rates and deviations per batch, including their closure times.
- Delivery quality: production planning accuracy using the forecast accuracy, the production schedule accuracy and the service level delivery.
- Customer quality: the complaint rate from the customer.
- Quality culture: building the foundation for quality by addressing more than 80 indicators, as well as cultural aspects related to quality, such as management commitment, company culture, preventive activities and continuous improvement.
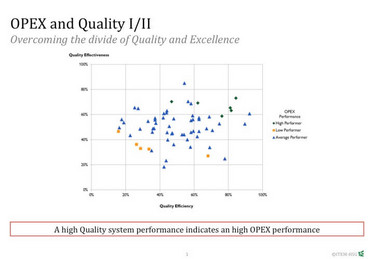
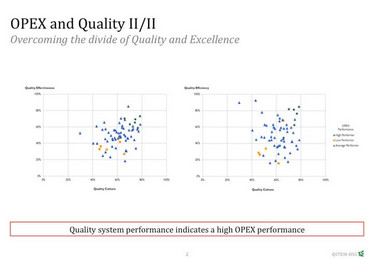
The framework allows you to examine your whole supply chain with quality-focused eyes, creating a reliable image of robust quality across the organization and its operations. We’ve been able to prove the potential benefits of OPEX by analyzing 300 data sets from pharmaceutical production sites using an algorithm that combines quality effectiveness and quality efficiency in relation to the OPEX performance of a production site. Sites with a high quality effectiveness and efficiency show a significant higher overall OPEX performance than others.
If you’d like to know more about the approach, you are more than welcome to analyze your own data at http://opexbenchmarking.com. We encourage more companies to overcome the divide of quality and excellence by reaping the benefits of both elements!
- FDA Draft Guidance, “Request for Quality Metrics,” (July, 2015). www.fda.gov
- T. Friedli et al., “Leading Pharmaceutical Operational Excellence,” Springer-Verlag Berlin Heidelberg (2013).
Christian Mänder is a researcher at the University of St.Gallen, Switzerland.
Thomas Friedli is a researcher at the University of St.Gallen, Switzerland.



















