Precision Biomanufacturing
Personalized medicine looks set to transform the biopharmaceutical landscape, and will demand significant changes in production methods. The demand for large-scale batch processes will decline – so what is the alternative? The UK’s new National Biologics Manufacturing Center is on a mission to answer that very question.
Jonathan Robinson |

As recently as 2009, the UK had the second strongest pharmaceutical industry in the world, but in recent years we have seen the sector falter. The UK is falling behind – fewer new drugs are being discovered here and drug manufacturing is drifting overseas. The UK government recognizes the value of the country’s significant expertise within the life sciences sector and wants to make sure we retain our leading position. To that end, a raft of government initiatives are being implemented to support UK life sciences companies and encourage them to develop and manufacture innovative new therapies within the UK. This includes the National Biologics Manufacturing Centre (NBMC), the Precision Medicine Catapult, the Cell Therapy Catapult and the Medicines Technology Catapult.
The Center for Process Innovation (CPI) is part of the UK’s High Value Manufacturing Catapult. We help to bring new technologies from universities and small companies into the market by providing the expertise, facilities and funding needed for successful translation. For example, we receive funding from the Advanced Manufacturing Supply Chain Initiative, as part of a consortium with big biopharma firms and a number of small companies with interesting technology. The aim is to streamline the development process for biologics by identifying unsuitable drug candidates earlier in the process. By allowing candidates to ‘fail early’, we hope to avoid abandoning candidates in Phase II or III clinical trials because they cannot be manufactured efficiently or aren’t compatible with the delivery device.
Making it personal
CPI recently opened the £38 million ($59 million) National Biologics Manufacturing Center (NBMC), with six key goals (see Table 1). In addition to optimizing current processes by introducing new techniques and technology, we are looking ahead to see what the next 20 or even 50 years might bring. Specifically, we want to address the increasing trend towards personalized medicine – therapies tailored to sub-populations, or even individual patients. With the advent of fast and efficient DNA sequencing, we are identifying increasing numbers of genetic subtypes of diseases. Breast cancer is a good example; treatment tailored to the hormone receptor and HER2 status of breast tumors is already well established, and as gene sequencing technology has advanced, more and more markers are being discovered.
As personalized or precision medicine becomes more prominent, it will start to challenge longstanding industry supply chain models. We will not be mass-producing these drugs to treat thousands of patients. Instead, patients will be tested for specific genetic or molecular markers and prescribed a drug to match their individual biological characteristics, which means more patient cohorts and smaller quantities of better tailored drugs. For the bioprocessing supply chain, this is a radical change. The large-scale batch processes of today will no longer meet society’s needs.
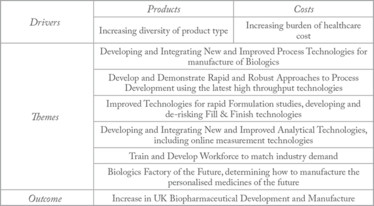
Table 1. CPI Biologics Strategy.
Miniaturized medicine
If personalized medicine takes off in the way we expect it to, what will be the impact on supply chains and what technologies will we need? Can we miniaturize production so that instead of scaling up, we can scale out? We hope to answer these questions with our Factory of the Future project, to understand how to manufacture smaller, more diverse materials by scaling down processes and making it easier to switch production between different products.
One solution is for biologics manufacturing to go mobile. Traditional bioprocessing is divided into separate unit operations (chromatography, filtration, and so on) but in the factory of the future I anticipate we will integrate many steps into single small-scale units that can be moved to the patient. By containing the process within a single piece of equipment you remove the need to work in vast clean rooms and make it possible to work anywhere.
Such small-scale integrated machines could produce medicines in a hospital or pharmacy setting to match the individual patient’s needs. Plus, mobile manufacturing units can be quickly added or repurposed to create a modular facility with much greater flexibility. Single use components are already well established in the industry and could be incorporated into the manufacturing unit to make it easy to switch production from one drug to another, improve flexibility and bypass costly clean-down steps.

On-line analytics
Analytics are another area where we expect the factory of the future to look very different to today’s facilities. Currently, analysis is typically conducted off-line – not ideal if you want to manufacture the product quickly on a small scale. Instead, we need to analyze the product as it’s produced, and so a major focus for CPI is on-line analytics for real-time measurement and control.
CPI is agnostic – we must consider all available technology that can help us achieve our goal. We encourage different manufacturing sectors to share ideas and technology, and we may find that the technology we need is already in use elsewhere. For example, the diagnostics industry already uses small microfluidic devices at point-of-care to measure analytes in samples. It is likely that elements of that technology could be applied for small-scale biologics production and analysis. In fact, a lot of the analytical technology needed is already in use in laboratories, and we are looking at how they can be brought online and incorporated into a discrete unit.

The road less travelled
Small-scale, disposable versions of current technologies, like bioreactors and chromatography columns, will certainly help get us started, but we also need some seriously fresh thinking. To fill the gap between what is currently available and what is needed, we are reaching out to innovative researchers at universities and small companies. For example, there is a lot of interest in small-scale continuous manufacturing technology and we are kicking off various projects to help bring that to market. Running continuous processes at a smaller scale is particularly challenging and raises a lot of questions – the role of CPI is to answer those technological questions to help the industry move forward.
The Factory of the Future project already has a healthy and growing consortium; the next step is to agree the goals with our partners so that we can start planning what we need to deliver and how we will test new technology. As with any innovation in the pharmaceutical industry, the biggest challenge is changing the current mindset. The industry is understandably cautious about moving away from tried and tested (and regulator-approved) technology. After all, shepherding a new product to market can be risky enough without adding new production methods and technology. However, doubters must see the benefit of taking the road less travelled – especially if they want to be at the forefront of personalized medicine, as current production methods are simply not suitable. The high cost of existing biologics has already caused controversy, and if we are to start producing drugs on a smaller scale, it’s imperative that the costs of production are decreased. Flexible, small-scale, high-throughput technology can help drive down the cost of developing biologics – and that can only make them more accessible.
Video: exploring the impact of precision medicine on biomanufacturing



















