Shining Excipient Star
Third place in our 2019 Innovation Awards was Colorcon’s StarTab – a starch-based excipient for direct compression. Colorcon’s Jayesh Parmar tells us more
What is your role at Colorcon?
In my role as General Manager for Formulation Technology, I have global responsibility for Colorcon’s excipient portfolio. This role involves identifying the unmet needs of the market and then working with our product development team to design concepts to address those gaps with innovative solutions. Before joining Colorcon, I worked as a formulator in the pharmaceutical industry and I’ve always had a keen interest in excipient science. In fact, from the very beginning, the role and importance of excipients in pharmaceutical dosage forms has blown me away! Excipients play a major role in pharmaceuticals; whether determining release kinetics or improving the flow properties or compressibility of a formulation. Many drugs are difficult to formulate due to their solubility, dose, or stability issues. Excipients are there to make it happen.
Why should pharma manufacturers pay careful attention to their choice of excipients?
As pharmaceutical companies strive to get their products to market in the shortest time, formulators want to develop cost effective yet robust formulations faster and ensure there are no barriers or delays with regulatory approval. To advance through the development milestones, it is imperative to select the right excipient – as well as a trusted supplier, who can provide technical support and understand the regulatory landscape across the world. When it comes to excipients, it can be surprising how regulations differ. Failing to select the right excipient and partner can easily derail the project plan...
What gaps in the market inspired the development of StarTab?
Development scientists have become increasingly interested in formulation simplification using a direct compression tablet manufacturing process, as it helps reduce development time and total cost in-use. Compared with wet or dry granulation methods, the direct compression process is simple and removes multiple manufacturing steps. Figure 1 shows a comparison of the steps involved in tablet manufacture.
Other excipients can be used for the direct compression process; however, the drawback tends to be that formulators need to add a flow-aid and a superdisintegrant. But adding a superdisintegrant adds cost and often results in stability issues because of the high affinity for moisture. Put simply, using StarTab in a direct compression formulation provides desired powder flow, tablet hardness, and disintegration, without the need for the addition of a flow aid or disintegrant.
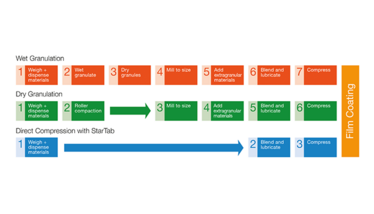
A comparison of the steps involved in tablet manufacture.
How does StarTab compare with other starch-based excipients?
Starch-based excipients have a long history of use in the pharmaceutical industry because of their inertness, capacity to remove free moisture in the formulation, and disintegrant properties. StarTab is a modified starch with unique particle shape and size distribution that improve its performance in direct compression while maintaining all the other benefits of starch as an ingredient. Multiple ingredients in a formulation may complicate development (and ultimately the manufacturing process); the effect of each component and its interaction with the API must be considered. StarTab allows scientists to simplify their formulation with a lower number of ingredients and still achieve the desired properties. StarTab also plays an important role in enhancing the stability of moisture sensitive APIs because of its low water activity.
What were the biggest challenges when developing StarTab?
In the eye of the regulator, we had to design an excipient that was novel but not “new” – a significant challenge. And so, throughout development, we had to ensure the product specification met the regulatory requirements without compromising on functionality. From a performance view, the product was developed to produce excellent flow and tablet hardness with fast disintegration – a challenging combination.
How have customers responded?
The market has responded positively! Companies are selecting StarTab for new formulation development projects. And many customers are even changing their existing wet granulation formulation technology to direct compression using StarTab.
What are the company’s plans for the remainder of 2020?
Despite the challenges related to COVID-19, we continue to innovate. We are bringing to market SoteriaRx, a new platform of on-dose authentication technologies and detection services for the authentication of medications. The overall aim is to help protect patients from counterfeit medicines and to uphold brand integrity. We believe the digitalization of medicines represents a major step forward in the fight against unauthorized and illegitimate pharmaceutical production and distribution.

Making great scientific magazines isn’t just about delivering knowledge and high quality content; it’s also about packaging these in the right words to ensure that someone is truly inspired by a topic. My passion is ensuring that our authors’ expertise is presented as a seamless and enjoyable reading experience, whether in print, in digital or on social media. I’ve spent fourteen years writing and editing features for scientific and manufacturing publications, and in making this content engaging and accessible without sacrificing its scientific integrity. There is nothing better than a magazine with great content that feels great to read.



















