The Evolution of Operational Excellence
In today’s competitive market, OPEX is – or at least should be – a priority for all pharmaceutical manufacturers. Looking back on just a decade of progress, what have we learned and where do we go next?
The history of operational excellence (OPEX) in the pharmaceutical industry is short; the first serious OPEX initiatives were only launched about 10 years ago. Before that, the pharmaceutical industry was reluctant to put OPEX and continuous improvement on its agenda, in part because of the regulatory environment but also because it was not suffering from the same cost pressures facing other industries, such as the automotive and electronics sectors, which were forced to adopt OPEX earlier.
The discussion about a more scientific approach to pharmaceutical manufacturing only really began at a US FDA scientific advisory board meeting towards the end of 2001. The agency was facing an increasing number of post-approval manufacturing amendments at the time, making it tough to fulfill their review and inspection obligations. It became apparent that the industry did not truly understand its own manufacturing processes and that there were gaps in the science needed to gain useful knowledge. Current good manufacturing practices (cGMP) were being driven more by experience than sound science, which raised concerns, as did the overly risk-averse nature of industry and regulators.
At the same meeting, Doug Dean and Francis Brutton from PriceWaterhouseCoopers presented a rather bleak analysis of the status of pharmaceutical manufacturing and identified the root causes:
- Processes that were neither fully understood nor suitable for commercial scale were being transferred from the laboratory to manufacturing.
- Lengthy and elaborate new product introduction exercises generated data but failed to provide critical information.
- 50 percent of production costs were locked in before Phase III had begun.
- Process inefficiencies were “institutionalized”; companies were reluctant to put in the time to gain deeper process understanding.
Both the industry and the FDA were well aware of the deficiencies in pharmaceutical manufacturing, and knew that to move forward they must encourage the use of innovative technologies to enhance process understanding and establish scientific, risk-based approaches to quality and regulatory processes. The FDA and the industry assembled a process analytical technology (PAT) Team to evaluate how they could further promote this concept. The PAT Team and Manufacturing Science Working Group agreed that things had to change:
“Pharmaceutical manufacturing operations are inefficient and costly. The cost of low efficiency is generally not understood or appreciated (e.g., manufacturing costs far exceed those for research and development operations). Low efficiency is predominantly due to ‘self-imposed’ constraints in the system (e.g., static manufacturing processes, focus on testing as opposed to quality by design, approach to specifications based on discrete or the so called ‘zero tolerance’ criteria, a less than optimal understanding of variability, etc.). These constraints keep the system in a corrective action mode. Continuous improvement is an essential element in a modern quality system and it aims at improving efficiency by optimizing a process and eliminating wasted efforts in production. In the current system continuous improvement is difficult, if not impossible (1).”
In response, the FDA shifted from its position of focusing on product purity and potency as its measure of quality, towards a regime that focused on the actual physical manufacturing processes (2). The idea was that a more thorough understanding of the processes would lead to more predictable and efficient manufacturing. In August 2002, the FDA announced a significant and bold initiative: Pharmaceutical cGMPs for the 21st Century. The aim was to encourage the early adoption of new technological advances by the pharmaceutical industry, to base regulatory review and inspection policies on state-of-the-art pharmaceutical science, and to facilitate the use of modern quality management systems. Risk-based approaches would focus both industry and agency attention on critical areas and incorporate enhanced quality system approaches into the agency’s business processes (3).
The FDA also gave details of what it termed the “desired state” – its vision for pharmaceutical manufacturing:
- Product quality and performance assured by design of effective and efficient manufacturing processes.
- Product specifications based on mechanistic understanding of how formulation and process factors impact performance.
- Continuous improvement approaches, with innovative use of new technology as desired.
Subsequent FDA activities were all based on the same underlying idea: to modernize the science base for pharmaceutical manufacturing and quality management (4).
OPEX evolution
Comparing today’s management of OPEX with the first early efforts, we can distinguish three evolutionary steps (see Figure 1). First, there was the “Pre-OPEX” phase that lasted until the late 1990s. Next came the learning – or “Best-Practice Transfer” – phase, which gave way to today’s “Transformation” phase. Looking towards the future, we hope to see the rise of a fourth phase: “Integrated Operations Systems,” where we move forward from simply using the tools of OPEX, by integrating the concepts into all aspects of manufacturing – and beyond (5). In our opinion, some pharmaceutical companies that use advanced OPEX programs are already on the threshold of entering the fourth phase – but they certainly didn’t get there overnight. Pharma companies currently launching programs can benefit from these experiences and essentially skip straight to the transformational phase.
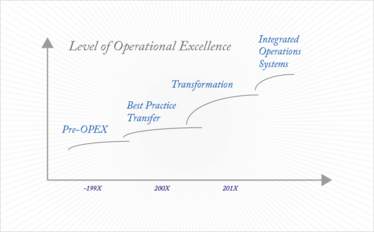
Figure 1. Pathway to operational excellence in the pharmaceutical industry
Pre-OPEX: it’s all about cGMP
In the late 1990s, pharmaceutical production was determined by one central force: the regulatory framework, predominantly based on cGMP guidelines. While compliance and finished product quality were the credo of pharmaceutical manufacturing, other practices to improve efficiency and flow were deemed irrelevant to or even incompatible with cGMP. The final product quality might well be excellent, but it was not based on well-understood and efficient processes (6). In fact, pharmaceutical manufacturing was characterized by a high number of rejected batches, lengthy laboratory tests, an extraordinarily high number of inspections and slow feedback loops for subsequent batches (5).
Best Practice Transfer: isolated OPEX applications
At the beginning of 2000, increasing cost pressure, the awareness of inefficiencies in the manufacturing area and the aforementioned regulatory initiatives prompted individual manufacturing sites to start experimenting with performance improvement tools, mostly originating from programs such as Six Sigma, Lean or Technical Performance Measurement. The main purpose was to increase efficiency by “doing things right first time”. This OPEX phase is characterized by individual projects dealing with specific tools and practices, guided by experts or external consultants (5). The focus was on tools, not people.
Transformation: company-wide initiatives and programs
What became obvious during the best practice transfer stage was that ideas often stalled, leading to missed opportunities. The approach was too technical and neglected the impact of the people involved in the processes. What was needed? Well, active engagement from top management, changes to the organizational setup and a change management program that actively engaged every single worker in the plant. With these missing pieces in place, sustainable implementation of pharmaceutical OPEX programs was finally possible.
Integrated Operations System: beyond the tools
How can companies reach the fourth and final stage of OPEX evolution? We believe there are a number of crucial aspects (5):
- The many different initiatives in leading pharmaceutical companies should be bundled into an umbrella program, aligning all key activities for improvement of operational competitiveness.
- New and improved practices will be developed and implemented. These practices will be generated internally, so they will rely on effective sharing of knowledge across the organization.
- OPEX will not be limited to practices and standard routines. The mindset to affect change and make improvements should prevail on all levels of the company.
- OPEX and performance should be seen in a wider context, and not be confined to the boundaries of an organization.
- OPEX will be integrated with quality management. The modern approach to quality is systems oriented, just like the OPEX program. Therefore, a properly planned and executed OPEX program should also be a measure of the effectiveness of the quality systems. On the other hand, overzealous OPEX programs could negatively impact quality. Finding a way to tackle this challenge is one of our key areas of research right now. In the future, OPEX and quality will not be two separate discussions.
Measuring the impact of OPEX
At the Institute of Technology Management at the University of St. Gallen, we assess the impact of OPEX using St. Gallen OPEX Benchmarking to show the individual performance of pharmaceutical production sites. Today, we have more than 270 pharmaceutical manufacturing sites from around 100 different companies in our database.
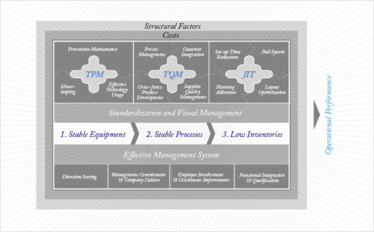
Figure 2: Structure of the St. Gallen operational excellence model. (TPM: total productive maintenance, TQM: technical quality management, and JIT: just-in-time)
Figure 2 gives an overview of the key elements in our Operational Excellence Benchmarking model. It measures performance by using production-specific key performance indicators (KPIs) that are closely linked to the technical sub-system (total productive maintenance (TPM), technical quality management (TQM) and just-in-time (JIT)) and the effective management system. With its focus on achieving the goal of “one-piece flow” and minimal buffer inventory, the JIT concept requires stable and robust processes. TQM complements JIT by creating a less variable and more stable manufacturing process that, in turn, reduces the need for safety stock buffers. In mass production, the breakdown of a machine usually does not create a sense of urgency; the maintenance department is scheduled to fix it while inventory keeps operations running. However, in a JIT environment, equipment breakdowns will soon lead to production downtimes. Hence, the concept of TPM, in which everyone learns how to clean, inspect and maintain equipment, becomes a crucial element of a truly excellent production environment (see Figure 3). You cannot build a stable process based on unstable equipment. Figure 2 also shows some key factors for success of the underlying effective management system, with a short summary of the relevant indicators from the St. Gallen OPEX benchmarking (7).
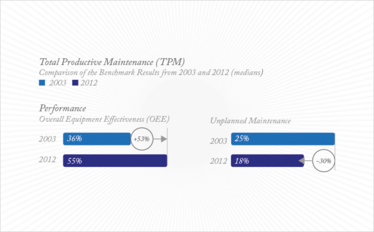
Figure 3: Performance improvements in total productive maintenance (8).
Our benchmark shows an improvement in performance over the last 10 years in both effectiveness and efficiency. For example, looking at TPM, we saw a 53 percent increase in overall equipment effectiveness and a 30 percent decrease in unplanned maintenance between 2003 and 2012 (8).
Another positive finding from our benchmarking is that the number of KPIs that companies are able to measure has increased significantly during the past year. Today, most companies can deliver every KPI we request in our benchmarking questionnaire. Clearly, pharmaceutical companies are thinking more about how to continuously enforce their OPEX activities and create a culture of continuous improvement within their organization.
OPEX is one of the most significant developments in pharmaceutical manufacturing in the last decade. The obvious benefits – streamlining processes and cutting costs – mean that increasing numbers of companies around the world are embracing OPEX. But there are a host of less obvious benefits too: a culture of continuous improvement, teamwork, employee involvement, and a process and systems approach to problem solving, to name just a few. Successful OPEX programs not only improve manufacturing by reducing defects and saving cost, but in the long-run also have an enormous impact on drug quality.
- FDA, “The PAT Team and Manufacturing Science Working Group Report: A Summary of Learning, Contributions and Proposed Next Steps for Moving towards the ‘Desired State’ of Pharmaceutical Manufacturing in the 21st Century” (2004).
- G. Clark, “FDA’s PAT initiative”, Pharm. Tech. Europe 16 (10), 34 (2004).
- FDA, Final Report “Pharmaceutical CGMPs for the 21st Century – A Risk-Based Approach” (2004).
- T. Friedli and J. Werani, “The History of OPEX in the Pharmaceutical Industry”, Leading Pharmaceutical Operational Excellence - Outstanding Practices and Cases, s27-34 (Springer Verlag, Berlin, 2013).
- T. Gronauer, T. Friedli, and M. Goetzfried, “The Roadmap to Operational Excellence: Pattern and Elements of OPEX Programs”, The Pathway to Operational Excellence in the Pharmaceutical Industry - Overcoming the Internal Inertia, s175-199 (Cantor Verlag, Aulendorf, 2010).
- P. Basu, “The Future of Pharmaceutical Manufacturing: QbD & OE re-inforcing one another”, The Pathway to Operational Excellence in the Pharmaceutical Industry - Overcoming the Internal Inertia, p. 324 (Cantor Verlag, Aulendorf, 2010).
- T. Friedli and D. Bellm, “OPEX: A Definition”, Leading Pharmaceutical Operational Excellence - Outstanding Practices and Cases, s7-26 (Springer Verlag, Berlin, 2013).
- T. Friedli, N. Lembke, U. Schneider, S. Gütter: The Current State of Operational Excellence Implementation:10 Years of Benchmarking. In: Leading Pharmaceutical Operational Excellence - Outstanding Practices and Cases. Berlin : Springer Verlag, 2013, S. 35-58
Thomas Friedli is a researcher at the University of St.Gallen, Switzerland.
Christian Mänder is a researcher at the University of St.Gallen, Switzerland.



















