
Track & Chase
Serialization deadlines will shortly be upon us – but, rather than entering into a blind panic, consider the long term.
There is less than a year left to go until the February 2019 enactment of the EU’s Falsified Medicines Directive, a regulation that, amongst other mandates, necessitates the printing of unique product identification codes on all prescription units of sale and homogenous cases. EU countries that already have their own form of serialization or tracking in place (Belgium, Italy and Greece) have until 2025 to harmonize their approaches with the new EU system, but all other EU countries must comply with the 2019 deadline. The UK, despite Brexit, is also expected to keep in line with the requirements. For the US, it’s even tighter – serialization requirements for products under the Drug Supply Chain Security Act come into force in November 2018. The final stretch is well and truly here.
A clear strategy for pharma companies tackling compliance (particularly late movers) has been to partner with contract manufacturing organizations (CMOs) or contract packaging organizations (CPOs), as it lessens the pain of rolling out their own solutions. In many cases, CMOs and CPOs have been quick to assess and adopt serialization solutions and to promote their capabilities to potential new customers – and it goes without saying that those with solutions that go beyond basic requirements are in the driving seat. Rolling out a serialization solution is complicated for any pharma organization, but the complexities are far more tangled for contract organizations, who need to report to brand owners with different standards, expectations and requirements. As such, scalability and flexibility are essential because solutions must be configurable.
Baseline serialization solutions can typically be implemented in a few months, but it takes a bit longer if you want a solution that goes beyond short-term compliance needs. It’s crucial to bear in mind that serialization is not just about compliance – it can also offer other business benefits: data can be used for production improvements and personnel allocation, for example.
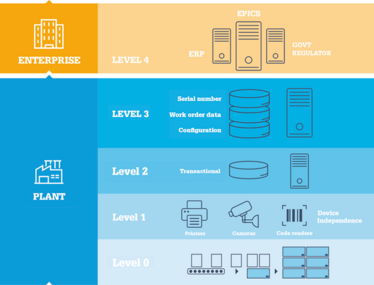
The levels of serialization.
Look ahead
In the US, serialization was initially due to be required by November 2017, but implementation was postponed for a year, giving those spearheading initiatives the opportunity to reassess their overall strategy. For instance, companies that had been rushing toward compliance via the most basic, expedient routes possible suddenly had time for some honest soul-searching.
With just over half a year to go until FMD, companies in Europe are at the last call for their own self-reflection. The biggest question to ask is, does the chosen solution represent a marriage of convenience, or a truly happy one? The most common flaw with many serialization solutions currently being embraced is that they are inherently shortsighted and, as a result, will be obsolete sooner rather than later. Remember the old adage “speed kills.” Rolling out a serialization solution is time-consuming, so you want to ensure you get it right the first time, rather than having to repeat the process a few years down the line. You need to think long term and remember that additional and more stringent serialization regulations will be phased in at some point. Turning again to the US as an example, the country’s Drug Supply Chain Security Act’s initial unit-level serialization mandate will be followed, in subsequent years, by other requirements including compatibility with government hubs and a rolling set of repackagers, distributors and drug dispensers, culminating in full supply chain traceability in 2023.
Why invest in a technology that will need to be replaced in five years? The serialization solution you choose should not be a one-time, single-use drop-in. Although time is short, you need to find the headspace to assess which solutions are scalable enough to meet rolling deadlines with phased-in requirements.
Heads up!
I was working with a company that needed to add bundling operations on seven packaging lines, but encountered a huge problem when they realized that a few of the lines couldn’t be updated using their existing serialization solution. Discovering such a problem only as you are rolling out your solution is pretty disastrous. (Fortunately, the company was able to correct the flaw in this case, but it still involved costly downtime and the requisite validation.)
Another issue that needs to be considered with serialization is cyber-security. As we move toward enterprise-level serialization, solutions are interconnected in a way that, in the age of “Internet of Things” can lead to vulnerabilities. Today, it is all too easy for criminals to take the cyber-backdoor into the networks of large companies and, once inside, wreak havoc. In May 2017, for example, the aptly named WannaCry ransomware hit a number of organizations, including large companies, hospitals and health services. Merck, Sharp & Dohme was also hit by the Petya cyber attack in 2017, which cost the company $135 million. For those pharma companies considering, for example, forgoing a previously planned transactional-only (Level 2) serialization system in favor of a more flexible enterprise-level solution (Level 4), the importance of proper encryption and virus protection cannot be overstated. Nor can the necessity of protected Cloud storage and secure data transmission between trading partners.
Communication standardization is also an issue. Part of the FMD is based on the formation of the European Medicines Verification System (EMVS), a pan-European system designed so that medicines can be verified at the point of dispensing. Upon FMD enactment, Marketing Authorization Holders that commercialize drug products distributed in the FMD’s jurisdiction will be required to upload their product serialization data to the European Hub. Reporting to the European Hub will entail generating and managing serialization data compliant with each target market, which involves certification as an official OBP (On-Boarding Partner) Gateway Provider – a designation that European pharma manufacturers would be wise to seek in serialization partners and vendors.
All abuzz
It’s inevitable: the vast majority of serialization solutions will reduce overall productivity. Whenever a new step is introduced to an extended process, such as pharma packaging, there will be some degree of slowdown. The important question is, to what degree? Many companies realize too late that their chosen serialization solutions significantly diminish production output. Unfortunately, some of them are already in too deep to mitigate this drop off, having installed systems on a multi-site, company-wide basis that would need to be ripped out and replaced, if significant speed gains are to be achieved. And that’s tens of millions of dollars in infrastructure alone – a cost simply not possible for many manufacturers.
Further Reading
What’s on the Box?
Six track-and-trace gurus give their insight into the upcoming deadlines. Read More
Serial Killer
Serialization is more than just adding a number to a box – data management will be the key challenge. Read More
Serialization Blind Spots
With all the complications of serialization, it’s easy to overlook a few key areas... Read More
To avoid this pitfall, there are two key buzz phrases: “hardware-agnostic solutions” and “beyond-compliance return on investment.” Simply put, hardware-agnostic solutions are less likely to become obsolete, and are more suited to making adjustments as production needs change or evolve. A process as intricate as serialization requires an outsized number of components to “play nice” with each other; these sorts of ongoing transactional relationships are best mediated by software. Beyond-compliance ROI alludes to ways of using the massive amount of data generated by serialization efforts to improve business practices (including the potential to reduce or even eliminate production slowdown caused by the system’s line incorporation). Solutions now exist that open up new horizons beyond regulatory compliance in terms of data analysis and machine learning tools, among other capabilities.
In actuality, the data pharma companies are required to gather, store and report to regulatory authorities can be valuable with the right tools. For example, some solutions can monitor overall equipment efficiency to pinpoint potential production bottlenecks. Associated systems track and predict production scenarios that allow companies to allocate the proper personnel for specific projects, maximizing individual strengths while limiting unnecessary overtime.
Time is dwindling for pharma companies, CMOs and CPOs to address both current and emerging regulations, while also minimizing the impact on production processes and productivity. But don’t panic and just opt for any system available – you’ve got to consider the long term and identify solutions that are flexible and scalable, while still being as painless as possible to deploy.
William Minaeff is Senior Project Director for Adents.
William Minaeff is Senior Project Director for Adents.



















